A kind of pyridone tetramycin and preparation method thereof and application in the preparation of anticancer drugs
A technology for medicines and medicinal salts, which is applied in the application field of pyridone phenteremycin and its preparation, and the preparation of anticancer drugs, and can solve the problems that the medicinal potential needs to be further tapped.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of Piericidinn pyride butterflycin
[0023] 1. The solid training of Streptomycess SP.Hberc-58855
[0024] Streptomycess SP.Hberc-58855 (preservation number CCTCC NO: M 2017186) is separated from mangrove mud. Yeast extracts 4g, glucose 4g, malt extract 10g, 30g of rough sea salt, 20g of agar powder, the remaining amount is water, pH 7.2-7.4; the preparation method is to mix the above components, sterilize 121 ° C, and pack.
[0025] 2. The amplification and fermentation of Streptomycess SP.Hberc-58855
[0026] Take a small amount of Streptomycess SP.Hberc-58855 diagonal bacteria for seed fermentation culture. Seed fermentation medium: each liter contains 20g of mannitol, soybean protein 胨 10g, soy oil 2.5g, hydrogen phosphate 0.35g. PH7.0, the preparation method is to mix each ingredients evenly and sterilize at 121 ° C. Use a 500ml triangle bottle 100 ml of seed fermentation medium, and the conditioning cultivation conditions are: 28 ° C, 120 rpm / mi...
Embodiment 2
[0029] Example 2: Structural identification of piericidinn pyrine butterflycin
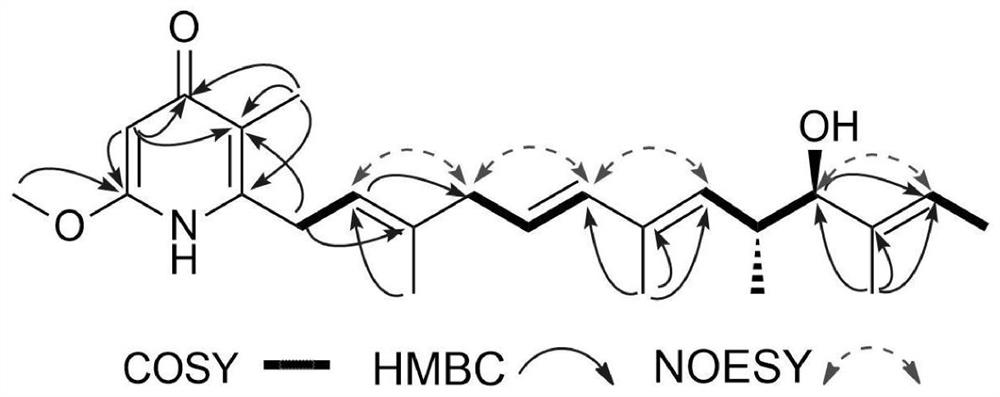
[0030] Perform structural analysis and testing of compound Piericidinn to get the following physical and chemical properties:
[0031] Piericidin n: light yellow oil; Uv (meoh) λ max (Logε) 211 (3.20), 223 (3.28), 227 (3.25), 238 (3.30) nm; CD (0.33mg / ml, meoh) λ max (Δε) 211 (1.04), 239 (-1.37), 254 (0.21) nm; 1 H and13 C NMR data is shown in Table 1; hresims M / Z 386.2696, [M+H] + (Calculated for C 24 H 36 NO 3 386.2690).
[0032] Based on HRRSIMS, the Piericidin N molecular formula is C as C 24 H 36 NO 3 (M / Z 386.2696 [M+H] + To. Its 1D-NMR nuclear magnetic data (Table 1) shows 7 methyl-based (including 1 methoxyl), 2 sub-methyls, 8 times methyl (including 6 hyperthyl methyl), and 7 seasons in seasons Carbon (containing 1 cymbal carbon). It is found that the only difference between Mer-A2026 B (J.ANTIBIOT.1995,48,103) with similar pink butterflyin compounds is found that the only difference is that...
Embodiment 3
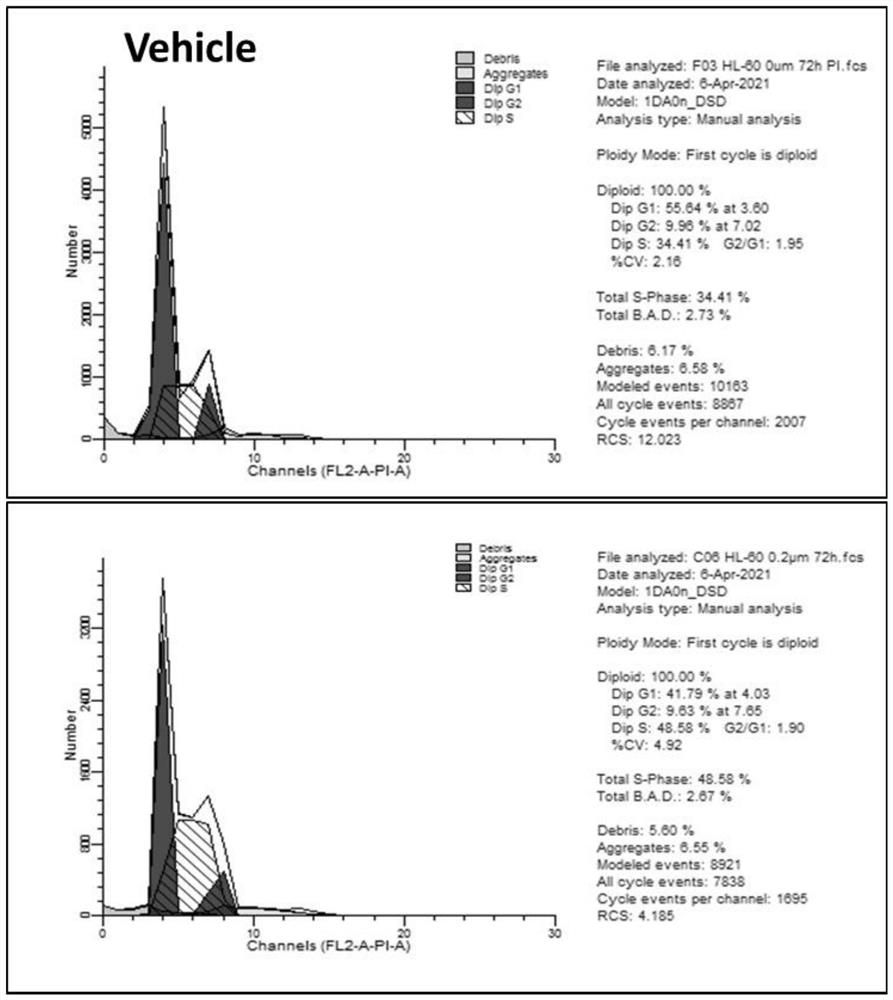
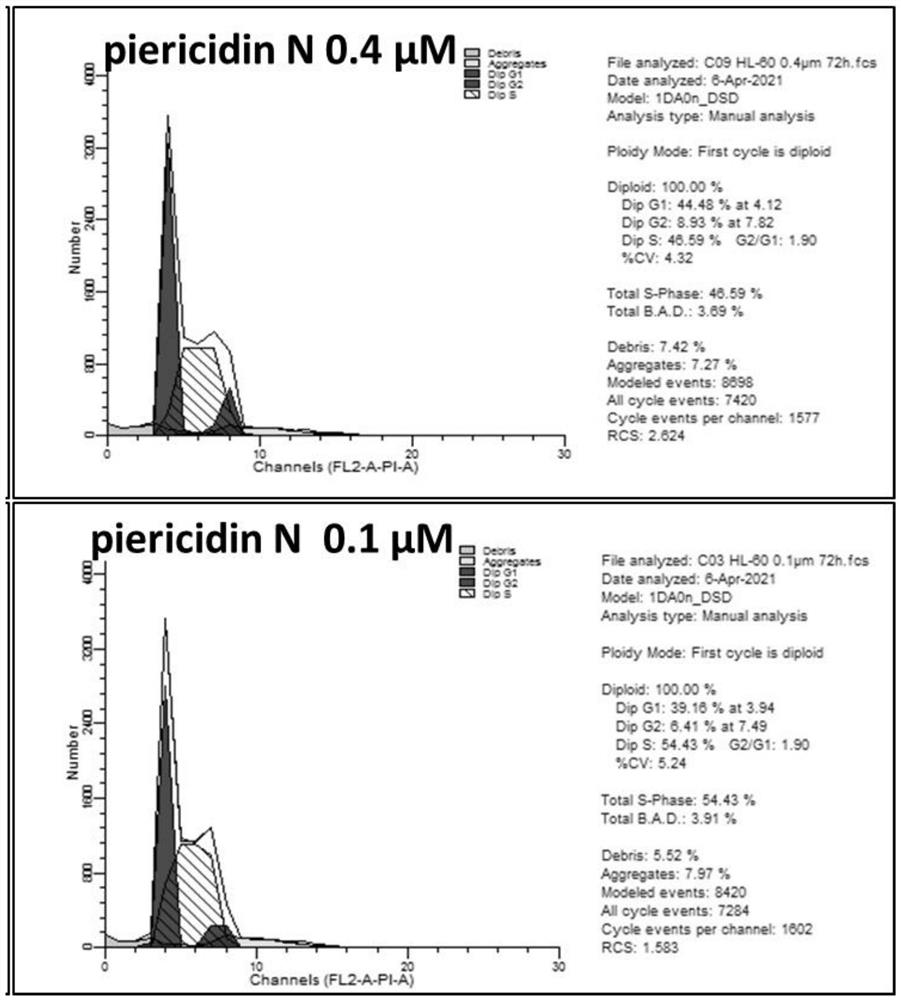
[0039] Example 3: pyride powder butterflycin Piericidinn's inhibitory activity of human cancer cells
[0040] 6 cancer cells are ordered at the Shanghai Academy of the Chinese Academy of Sciences: HL-60 cell strain (Cat#TCHU 23); K-562 cell strain (Cat#TCHU191); Molt-4 cell strain (Cat#TCHU224); 786-O Cat#TCHU186, Achn cell strain (Cat#TCHU199); OS-RC-2 cell strain (Cat#TCHU40). Cancer cell inhibitory activity experiments adopt CCK-8 testing method. Collect the number of cells, count the number of cells, and re -suspend the cells with a complete medium, adjust the cell concentration to the appropriate concentration (determined according to the test results of the cell density optimization test), inoculate the 96 -hole plate, and add 100 μl cell suspension per hole. Cells are 37 ° C, 100 % relative humidity, 5 % CO 2 The training box was incubated for 24 hours. Use a medium to dilute the compound to the appropriate concentration, and add the cells by 25 μl / hole. The final concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com