Application of moving point motor protein small molecular inhibitor to inhibition of tumor cell proliferation
A technology of tumor cell proliferation and motor protein, which is applied in the field of small molecule inhibitors of kinetochore motor protein in inhibiting tumor cell proliferation. issues such as stable phenotypes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1, the synthesis of target compound formula VII:
[0068]
[0069] (Formula XV) (Formula VII)
[0070] Add 1mmol 5a, the compound shown in 1.1mmol intermediate formula XV (its synthetic reference document Monatshefte fuer Chemie, 127 (5), 549-555,1996) in the three-necked flask, in 10ml THF, add 2mmol K under stirring 2 CO 3 , heated to reflux, monitored by TLC until the reaction was complete, cooled, filtered with suction, recovered the solvent under reduced pressure, dissolved the obtained residue in chloroform, washed with water, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was recovered by suction filtration, and the residue was recrystallized from isopropanol / dichloromethane to obtain the pure product of the target compound of formula VII.
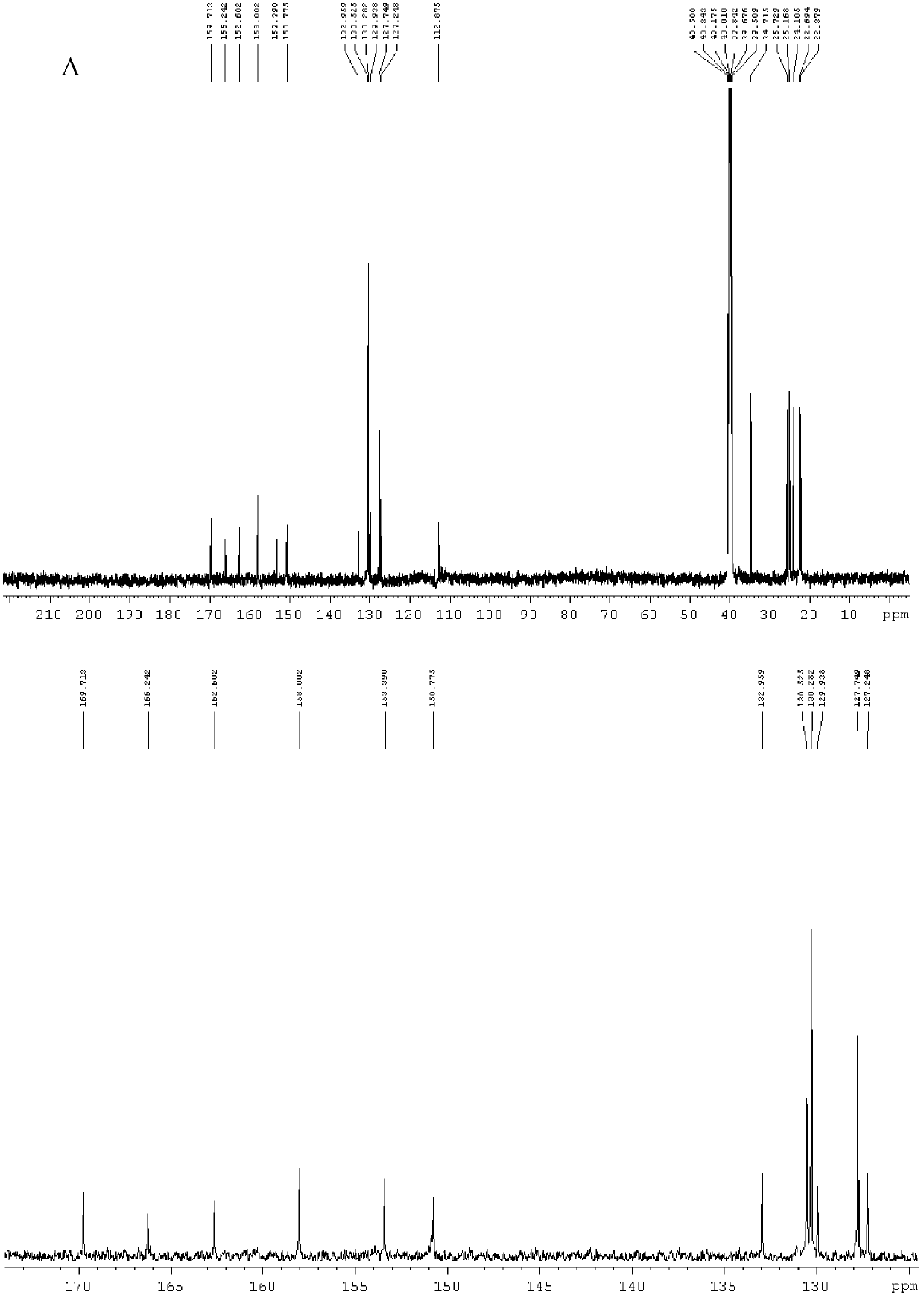
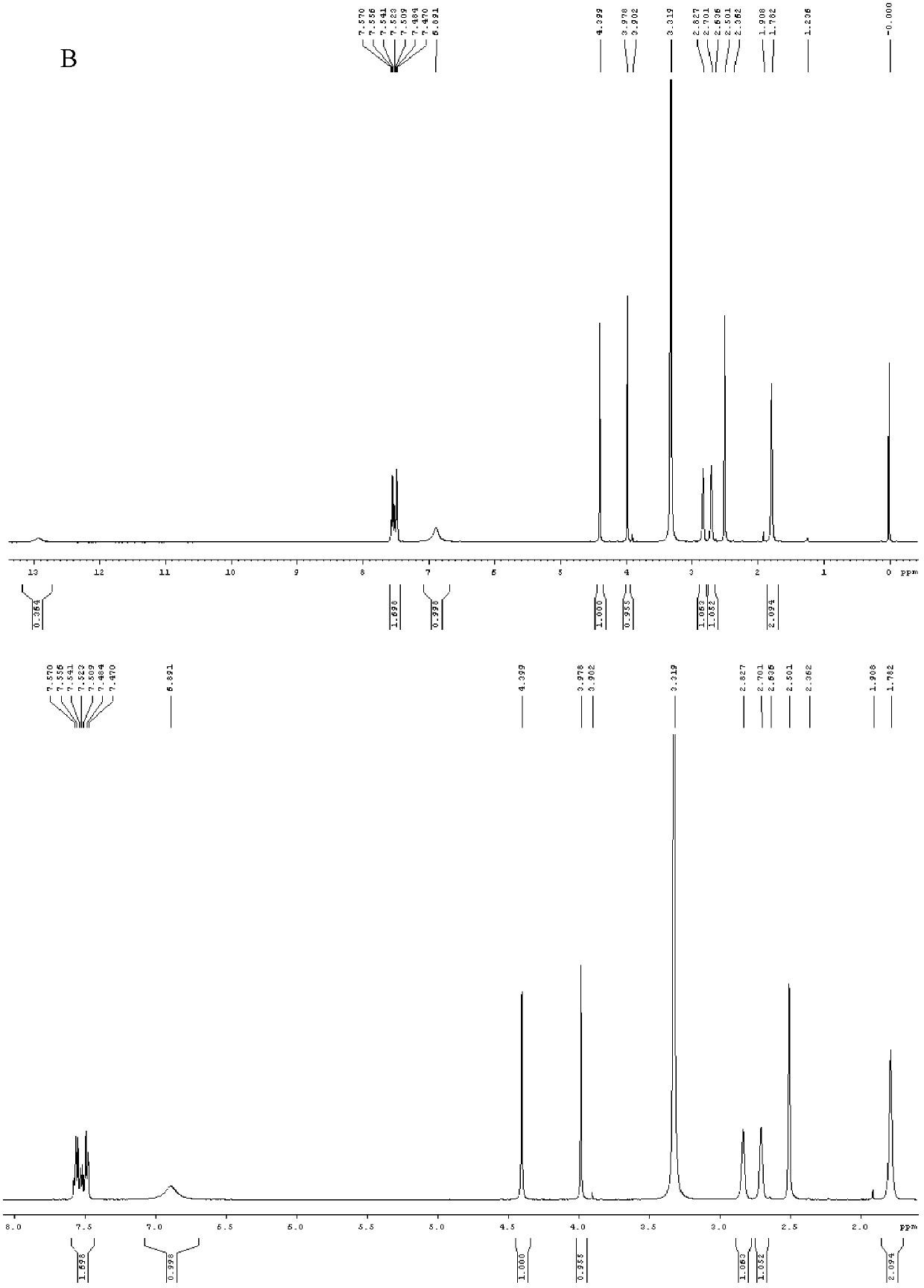
[0071] figure 1 It is the nuclear magnetic detection spectrogram of the pure product formula VII compound (named as Syntelin), A is the carbon spectrum, and B is the nitroge...
Embodiment 2
[0085] Embodiment 2, the synthesis of target compound formula III:
[0086]
[0087] (Formula XIV) (Formula III)
[0088] Add 1mmol 5b, the compound shown in 1.1mmol intermediate formula XIV (its synthetic reference Bioorganic & Medicinal Chemistry Letters 16 (17), 4444-4449, 2006) in the three-necked flask, in 10ml THF, add 2mmol K under stirring 2 CO 3 , heated to reflux, monitored by TLC until the reaction was complete, cooled, filtered with suction, recovered the solvent under reduced pressure, dissolved the obtained residue in chloroform, washed with water, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was recovered by suction filtration, and the residue was recrystallized from isopropanol / dichloromethane to obtain the pure product of the target compound represented by formula III.
[0089] Wherein, compound 5b is prepared according to the following steps:
[0090] 1) Synthesis of intermediate 1b:
[0091]
[0092] For the syn...
Embodiment 3
[0102] Embodiment 3, the synthesis of target compound formula VIII:
[0103]
[0104] (Formula XV) (Formula VIII)
[0105] Add 1mmol 5a, the compound shown in 1.1mmol intermediate formula XV (its synthetic reference document Monatshefte fuer Chemie, 127 (5), 549-555,1996) in the three-necked flask, in 10ml THF, add 2mmol K under stirring 2 CO 3 , heated to reflux, monitored by TLC until the reaction was complete, cooled, filtered with suction, recovered the solvent under reduced pressure, dissolved the obtained residue in chloroform, washed with water, washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was recovered by suction filtration, and the residue was recrystallized from isopropanol / dichloromethane to obtain the pure product of the target compound formula VIII.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com