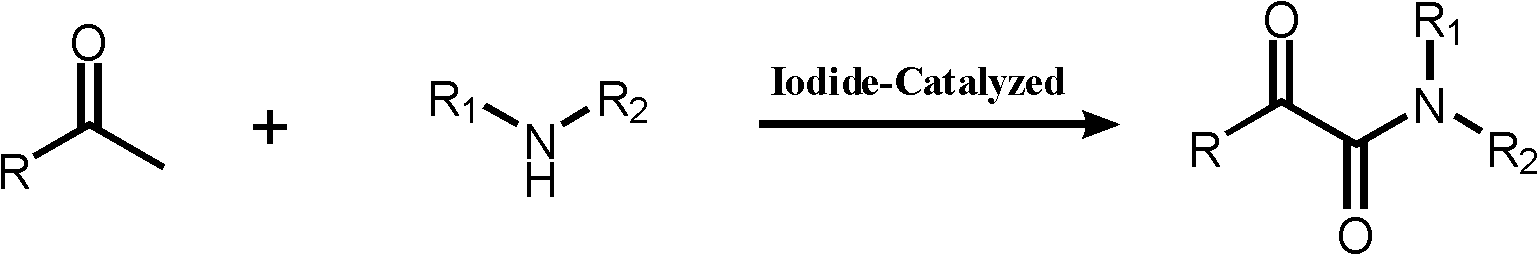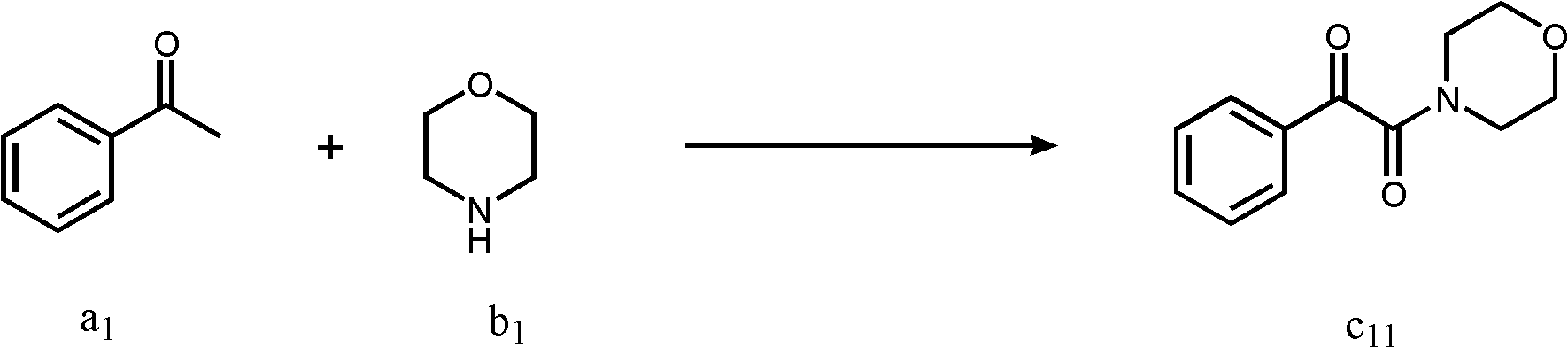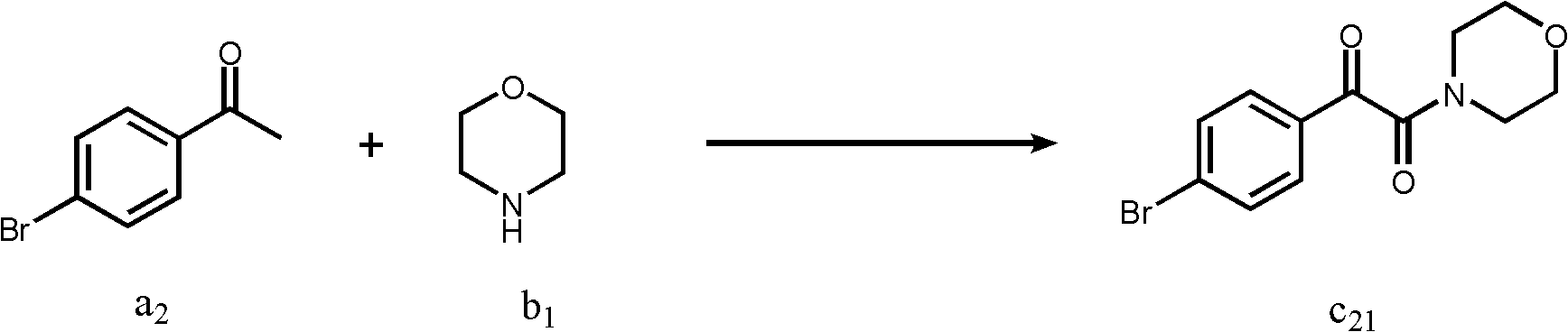Preparation method of alpha-keto amide
A technology of ketoamide and primary amine, which is applied in the field of preparation of α-ketoamide, which can solve the problems of cumbersome steps and achieve the effects of reduced pollution, excellent yield and short preparation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of preparation method of α-ketoamide, its reaction formula is as follows:
[0033]
[0034] The reaction bottle is filled with I 2 (1mmol), compound a 1 (2mmol), compound b 1 (2mmol), the solvent isopropanol 2mL. Then the system was stirred in air at room temperature for about 12 hours, then quenched by adding saturated sodium thiosulfate solution, extracted with ethyl acetate (2mL×3), then adsorbed with 100-200 mesh silica gel, and passed through 300- 400 mesh silica gel column eluting to obtain product c 11 , the yield was 90%. 1 H NMR (CDCl 3 , 400MHz): δ7.98-7.95(m, 2H), 7.68-7.64(m, 1H), 7.55-7.51(m, 2H), 3.81(t, J=4.0Hz, 2H), 3.79(t, J =4.0Hz, 2H), 3.66(t, J=4.0Hz, 2H), 3.39(t, J=4.0Hz, 2H); 13 C NMR (CDCl 3 , 100MHz): δ191.0, 165.2, 134.8, 132.8, 129.4, 128.9, 66.5, 66.4, 46.0, 41.4.; HRMS (ESI) m / z [M+H] + Calcd for C 12 h 14 NO 3 : 220.0968, found: 220.0975; IR(KBr, cm -1 ): v1681, 1645. The above detection data confirmed that the target...
Embodiment 2
[0036] A kind of preparation method of α-ketoamide, its reaction formula is as follows:
[0037]
[0038] The reaction flask was filled in sequence (CH 3 ) 4 NI (0.5mmol), compound a 2 (1 mmol), compound b 1 (2mmol), solvent dichloromethane 2mL. After stirring at room temperature in air for about 12 hours, the reaction was quenched by adding saturated sodium thiosulfate solution, extracted with ethyl acetate (2mL×3), then adsorbed with 100-200 mesh silica gel, and then passed through 300-400 mesh silica gel The product c was obtained by column elution 21 , the yield was 82%. 1 H NMR (CDCl 3 , 400MHz): δ7.83(d, J=8.6Hz, 2H), 7.68(d, J=8.6Hz, 2H), 3.79(t, J=4.0Hz, 4H), 3.67(t, J=4.0Hz , 2H), 3.38(t, J=4.0Hz, 2H); 13 C NMR (CDCl 3 , 75MHz): δ189.8, 164.7, 132.3, 131.7, 130.9, 130.3, 66.5, 66.4, 46.1, 41.5; HRMS (EI) m / z [M] + Calcd for C 12 h 12 79 BrNO 3 : 297.0001, found: 297.0007; Calcd for C 12 h 12 81 BrNO 3 : 298.9980, found: 299.0022; IR(KBr, cm -1): ...
Embodiment 3
[0040] A kind of preparation method of α-ketoamide, its reaction formula is as follows:
[0041]
[0042] The reaction flask was filled in sequence (CH 3 ) 3 PhCH 2 N 4 I (0.5mmol), compound a 3 (3mmol), compound b 1 (4mmol), solvent 1,1,1-trichloroethane 2mL. Then the system was stirred in air at room temperature for about 12 hours, then quenched by adding saturated sodium thiosulfate solution, extracted with ethyl acetate (2mL×3), then adsorbed with 100-200 mesh silica gel, and passed through 300- 400 mesh silica gel column eluting to obtain product c 31 , the yield was 83%. 1 H NMR (CDCl 3 , 400MHz): δ9.25(d, J=8.7Hz, 1H), 8.14(d, J=8.2Hz, 1H), 8.05-8.03(m, 1H), 7.93(d, J=8.1Hz, 1H) , 7.73-7.69(m, 1H), 7.63-7.55(m, 2H), 3.88-3.78(m, 4H), 3.67(t, J=4.0Hz, 2H), 3.44(t, J=4.0Hz, 2H ); 13 C NMR (CDCl 3 , 75MHz): δ193.4, 165.8, 136.0, 134.3, 133.8, 130.6, 129.3, 128.6, 128.1, 126.9, 125.5, 124.3, 66.41, 66.40, 46.1, 41.5; HRMS (ESI) m / z [M+H] + Calcd for C 16 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com