Dipeptide derivatives and their applications
A technology of uses and drugs, applied in the directions of dipeptide components, peptides, drug combinations, etc., can solve problems such as unsatisfactory treatment methods and lack of liver failure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
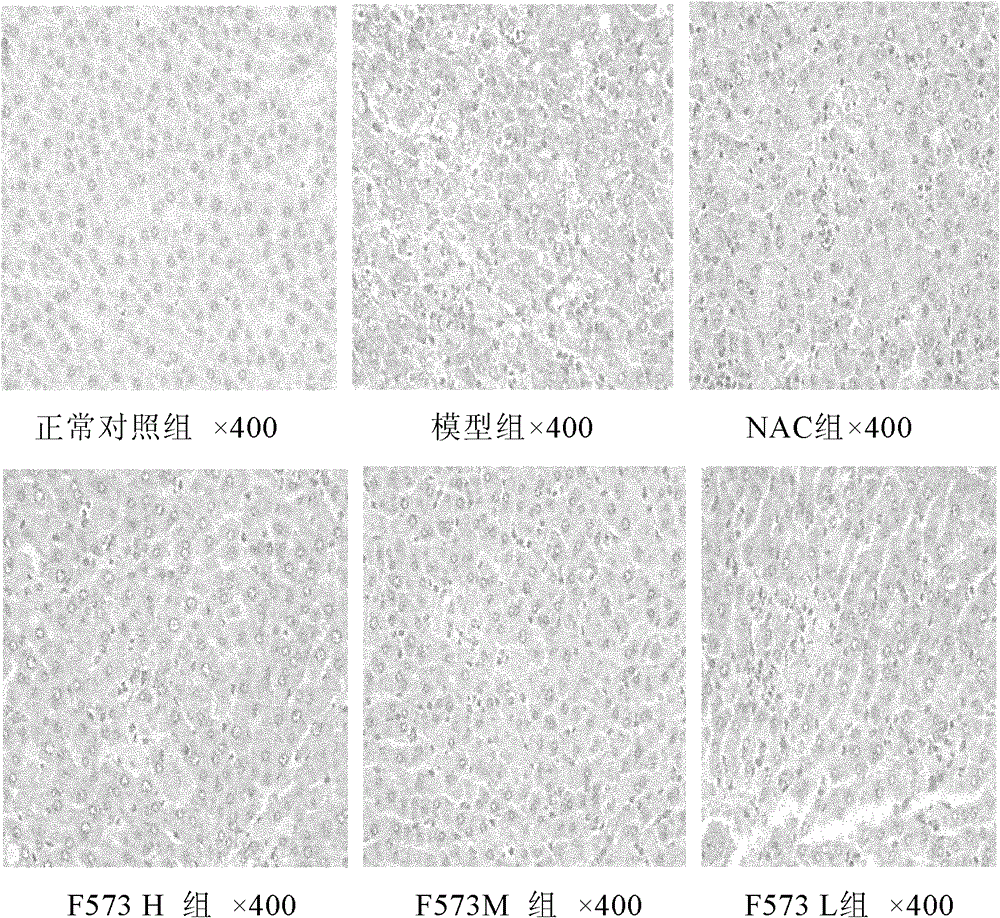
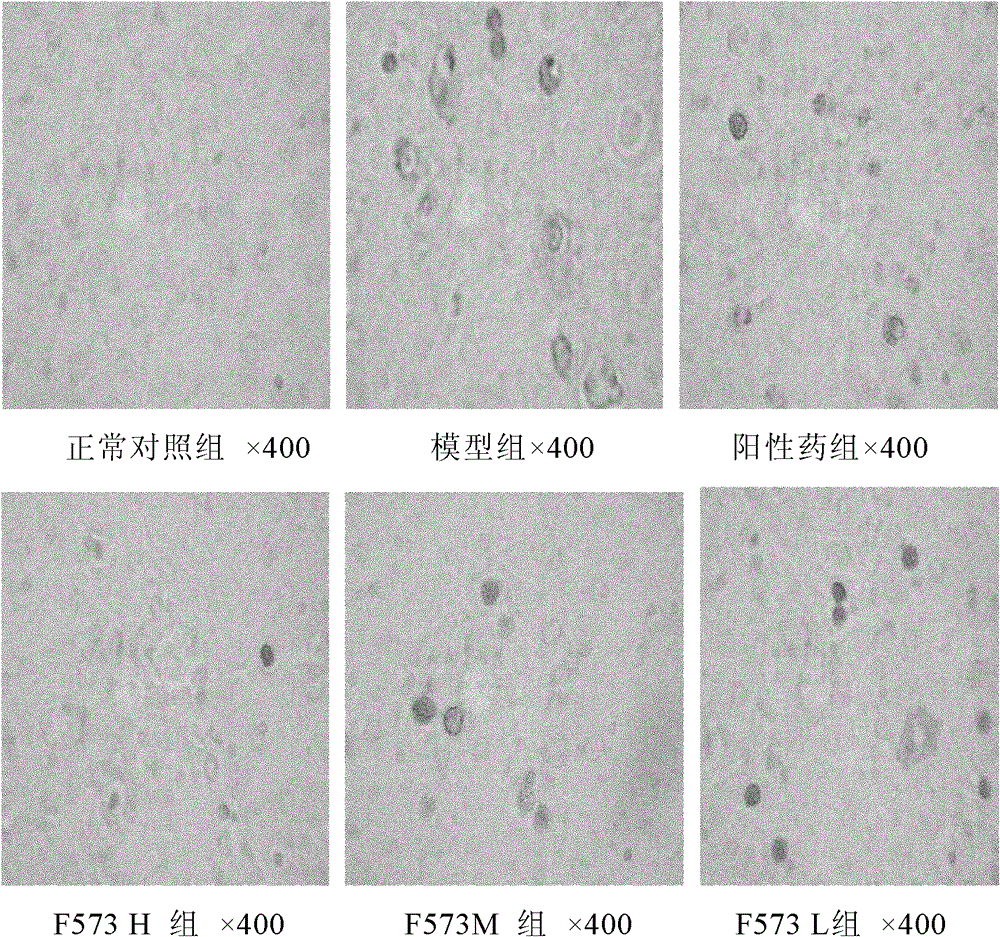
[0085] Give F573 after modeling
[0086] 1. Method
[0087] Wistar rats were randomly divided into 6 groups: normal control group, model group, positive drug N-acetylcysteine (NAC 155mg / kg) group and F573 high-dose (H 5.0mg / kg), medium-dose (M 2.5 mg / kg), low-dose (L 1.25mg / kg) groups, 10 rats in each group, except the blank group, each group was intraperitoneally injected (ip) with D-Gal500mg / kg and LPS 20μg / kg, and the model was established for 2 Hours later, each group ip corresponding NAC and F573, and the blank group was given the corresponding volume of solvent, namely:
[0088] ①Blank group: ip solvent (0.5% CMC physiological saline), ip 2ml / 200g according to body weight
[0089] ②Model group: ip D-Gal 500mg / kg and LPS 20μg / kg and ip solvent 1ml / 200g
[0090] ③NAC group: ip D-Gal 500mg / kg and LPS 20μg / kg+NAC 155mg / kg
[0091] ④F573H group: ip D-Gal 500mg / kg and LPS 20μg / kg+F573 5.0mg / kg
[0092] ⑤F573M group: ip D-Gal 500mg / kg and LPS 20μg / kg+F573 2.5mg / kg
[00...
Embodiment 2
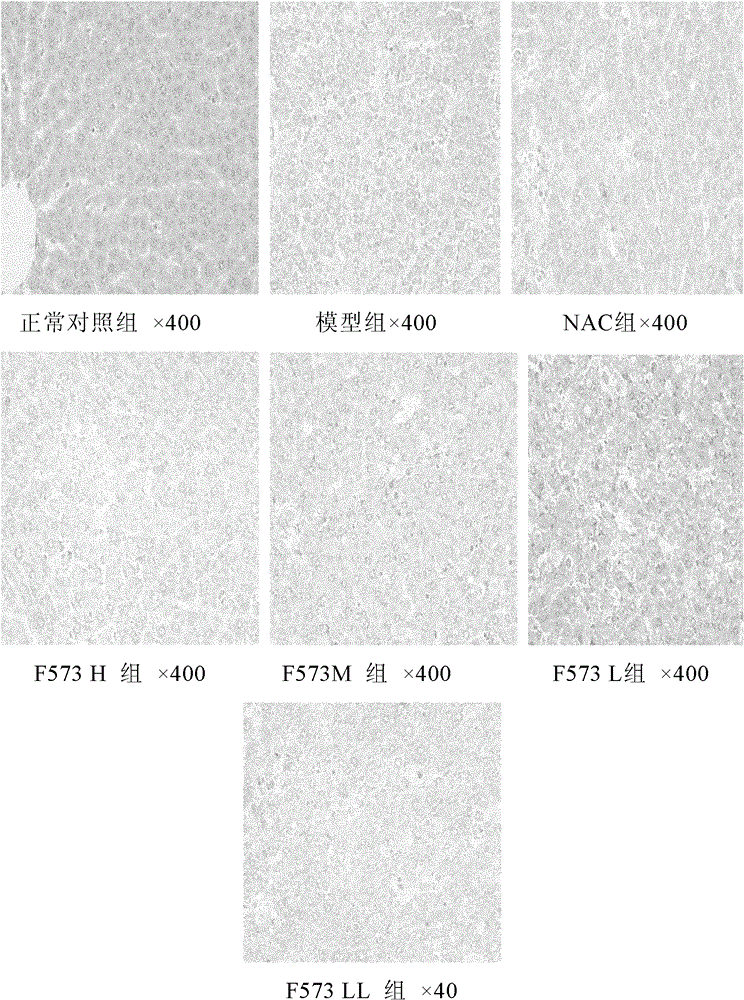
[0150] Modeling while giving F573
[0151] 1. Method
[0152] Repeat Example 1, the difference is: 70 Wistar rats are randomly divided into 7 groups: normal control group, model group, positive drug N-acetylcysteine (NAC 155mg / kg) group and F573 high dose (H : 5mg / kg), medium dose (M: 2.5mg / kg), low dose (L: 1.25mg / kg) and very low dose (LL: 0.625mg / kg) groups, 10 rats in each group, except the blank group In addition, the modeling drugs D-Gal 500 mg / kg and LPS 20 μg / kg were injected intraperitoneally (ip) in each group, and the corresponding NAC and F573 were administered in each group ip at the same time as the modeling (0 hour), and the corresponding volume of solvent was given to the blank group, namely:
[0153] ①Blank group: ip solvent (0.5% CMC physiological saline), ip 2ml / 200g according to body weight
[0154] ②Model group: ip D-Gal 500mg / kg and LPS 20μg / kg+ip solvent 1ml / 200g
[0155] ③NAC group: ip D-Gal 500mg / kg and LPS 20μg / kg+NAC 155mg / kg
[0156] ④F573H gr...
Embodiment 3
[0209] Give F573 before modeling
[0210] 1. Method
[0211] Repeat Example 1, the difference is: 4 hours before modeling, each group ip corresponding NAC, F573, and the blank group was given a corresponding volume of solvent, namely:
[0212] ①Blank group: ip solvent (0.5% CMC physiological saline), ip 2ml / 200g according to body weight
[0213] ②Model group: ip D-Gal 500mg / kg and LPS 20μg / kg+ip solvent 1ml / 200g
[0214] ③NAC group: ip D-Gal 500mg / kg and LPS 20μg / kg+NAC 155mg / kg
[0215] ④F573H group ip D-Gal 500mg / kg and LPS 20μg / kg+F573 5.0mg / kg
[0216] ⑤F573M group ip D-Gal 500mg / kg and LPS 20μg / kg+F573 2.5mg / kg
[0217] ⑥F573L group ip D-Gal 500mg / kg and LPS 20μg / kg+F573 1.25mg / kg
[0218] The experimental results showed that after the rats were treated with D-GalN / LPS, typical fulminant hepatic failure appeared, the levels of serum ALT, AST and T Bil increased sharply and significantly, and the cell apoptosis was significant, and the model was established.
[0219]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com