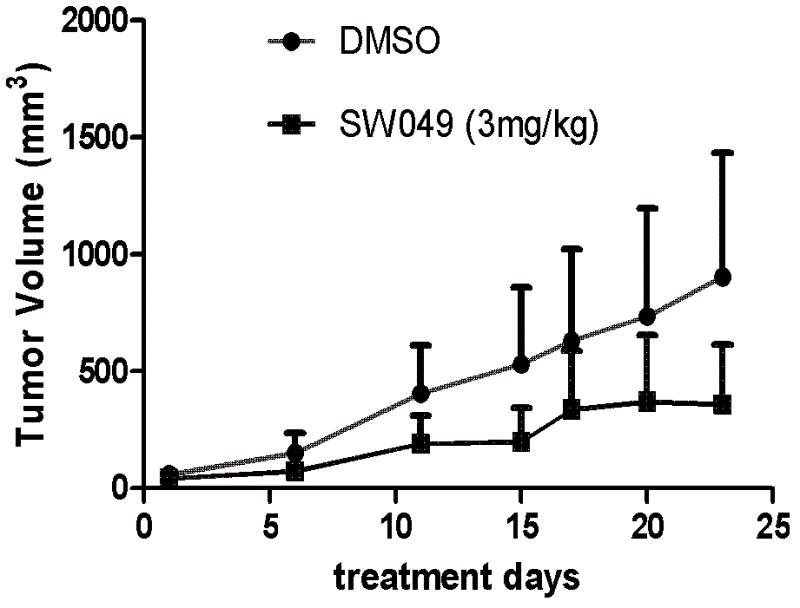
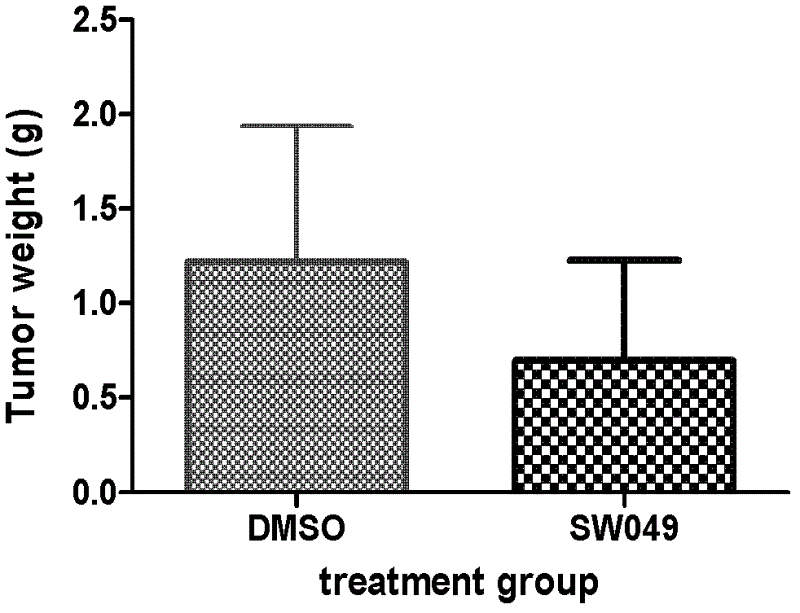
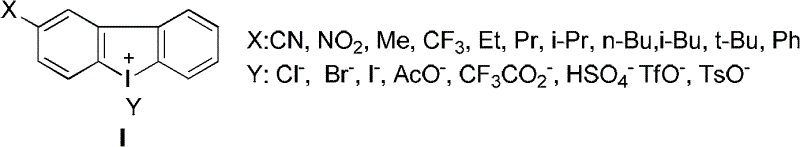Aromatic iodonium salts as NADH oxidase inhibitors and anti-tumor application thereof
A nicotinamide adenine and oxidase inhibitor technology is applied to the reduced nicotinamide adenine dinucleotide oxidase inhibitor aromatic iodonium salt and its anti-tumor application field, which can solve the problem of slow onset of anti-tumor drugs, slow onset of anti-tumor drugs, Easy to produce drug resistance, clinical use restrictions and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1, Synthesis of Compound 1:
[0033]
[0034] In an ice-water bath, slowly add the acetic acid solution (1.5mL) of raw material 1-a (50mg, 0.16mmol) to the mixed solution of potassium disulfate (52mg, 0.19mmol) in concentrated sulfuric acid (1mL) and acetic acid (1.5mL). ). After the addition was completed, the reaction solution was stirred under an ice bath for 30 minutes, and then stirred at room temperature for 3 hours. After the completion of the reaction, the reaction solution was poured into an aqueous sodium chloride solution with crushed ice, and the white precipitate obtained was collected by filtration, washed with ice water three times, and dried in vacuo to obtain compound 1 (25 mg, 45%). 1 HNMR (400MHz,) δ 8.54 (d, J = 8.2 Hz, 1H), 8.47 (d, J = 8.0 Hz, 1H), 8.39 (d, J = 9.0 Hz, 1H), 7.96 (d, J = 2.5 Hz, 1H), 7.77 (t, J=7.6 Hz, 1H), 7.58 (t, J=7.7 Hz, 1H), 7.22-7.15 (m, 1H), 3.91 (s, 3H). MS(ESI, m / z)309.0(M + ).
Embodiment 2
[0035] Example 2: Synthesis of Compound 2:
[0036]
[0037] To a dichloromethane solution (4 mL) of 1a-1 (50 mg, 0.16 mmol) was added m-chloroperoxybenzoic acid (75%, 45 mg, 0.19 mmol) and trifluoromethanesulfonic acid (43 uL). After stirring for 1 hour, the reaction solution was concentrated, and then ether (1 mL) was added. After 10 minutes, the precipitated solid was collected by filtration and dried to obtain solid compound 2 (45 mg, 61%). 1 H NMR (400MHz, d6-DMSO) δ 8.56 (d, J = 6.8 Hz, 1H), 8.21 (d, J = 7.7 Hz, 1H), 8.05 (s, 2H), 7.86 (s, 1H), 7.71 (s, 1H), 7.33 (d, J=8.8 Hz, 1H), 3.95 (s, 3H). 13 C NMR (100MHz, d6-DMSO) δ 161.7, 143.3, 141.5, 131.2, 130.6, 127.4, 121.8, 118.4, 111.6, 110.5, 56.2. MS(ESI, m / z)308.9(M + ).
Embodiment 3
[0038] Example 3 Synthesis of Compound 3:
[0039]
[0040] 3 was prepared according to the synthetic strategy of compound 2. 1 H NMR (400MHz, d6-DMSO) δ8.55 (dd, J=7.9, 1.1Hz, 1H), 8.23 (d, J=8.2Hz, 1H), 8.06 (dd, J=14.8, 5.9Hz, 2H) , 7.89-7.81 (m, 1H), 7.73-7.67 (m, 1H), 7.50 (d, J = 8.0 Hz, 2H), 7.32 (dd, J = 9.1, 2.8 Hz, 1H), 7.12 (d, J = 7.8 Hz, 2H), 3.95 (s, 3H), 2.29 (s, 3H). 13 C NMR (100MHz, d6-DMSO) δ 161.6, 145.5, 143.3, 141.5, 137.7, 131.3, 131.0, 130.6, 128.1, 127.3, 125.5, 121.8, 118.3, 111.6, 110.5, 56.1, 20.7. MS(ESI, m / z)308.9(M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com