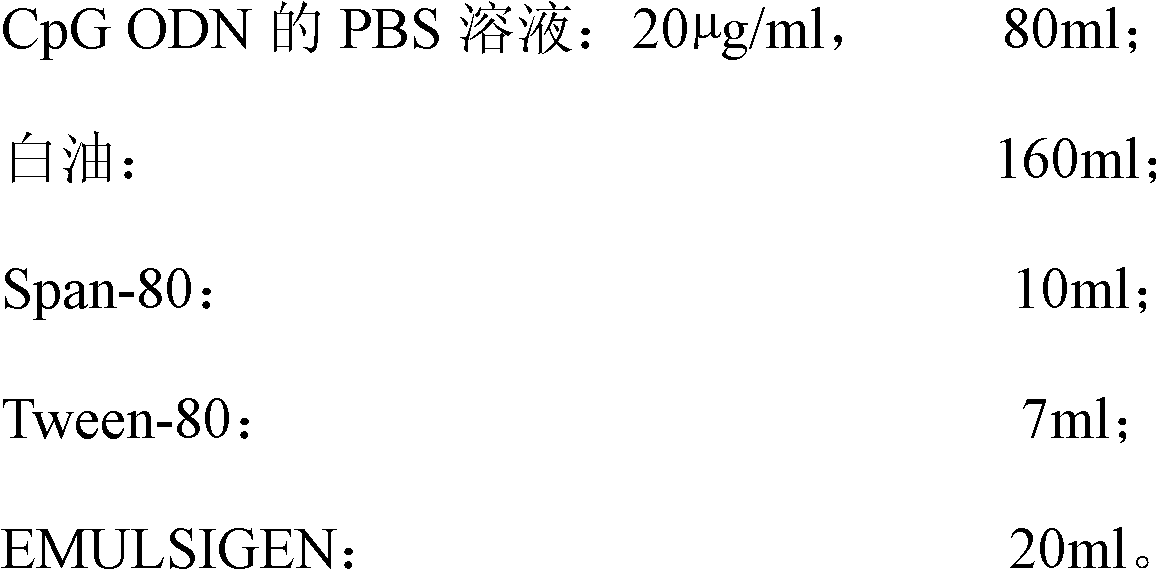High-efficiency CpG preparation, preparation method thereof and application
A preparation and high-efficiency technology, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, oil/fat/wax non-active ingredients, etc., can solve problems such as poor stability, harsh storage conditions, and decreased drug efficacy, and achieve Reduced usage, low preparation cost, effect of reduced usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 CpG preparation
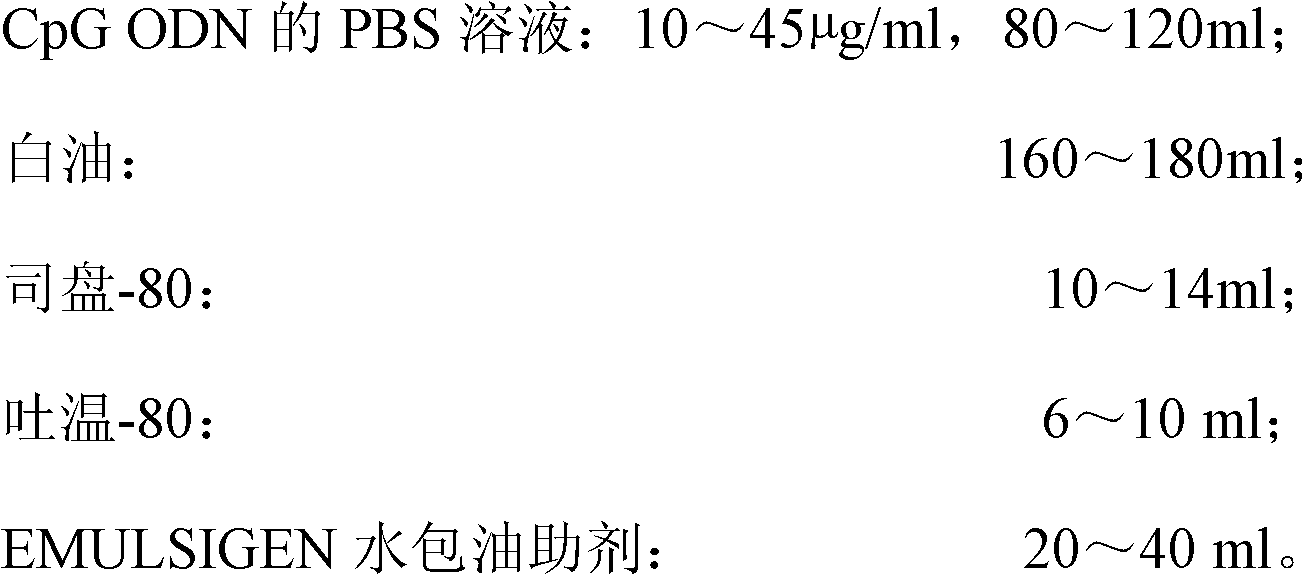
[0031] Prepare raw materials according to the following ratios:
[0032]
[0033] The PBS composition is: by weight percentage, 0.6% NaCl, 0.01% KCl, 0.0124% Na 2 HPO 4 , 0.014% KH 2 PO 4 Soluble in water; damp heat autoclave: 115°C, 15min.
[0034] (1) After CpG ODN was synthesized by TAKARA Company, it was prepared into 20 μg / ml with sterile PBS solution and stored at -15°C for later use;
[0035] (2) Take 160ml of white oil, 10ml of Span-80 and 7ml of Tween-80, mix well with a stirrer at 200rpm / min for 4min, and bathe in water at 60°C for 20min;
[0036] (3) Take 20ml of EMULSIGEN (EM) and the solution obtained in step (2), stir with a stirrer at 200rpm / min for 4min to mix evenly, and store at 4°C for later use;
[0037] (4) Add 80ml of the CpG ODN solution obtained in step (1) into the solution in step (3), then stir with a stirrer at 600rpm / min for 4min to emulsify and mix evenly, and place at room temperat...
Embodiment 2
[0040] Prepare raw materials according to the following ratios:
[0041]
[0042] The PBS composition is: by weight percentage, 0.7% NaCl, 0.015% KCl, 0.013% Na 2 HPO 4 , 0.0145% KH 2 PO 4 Soluble in water; damp heat autoclave: 117°C, 19min.
[0043](1) After CpG ODN was synthesized by TAKARA Company, it was prepared with sterile PBS solution to 22 μg / ml, and stored at -18°C for future use.
[0044] (2) Take 170ml of white oil, 12ml of Span-80 and 6ml of Tween-80, mix well with a stirrer at 250rpm / min for 5min, and bathe in water at 65°C for 25min.
[0045] (3) Take 25ml of EMULSIGEN (EM) and the solution obtained in step (2), stir with a stirrer at 250rpm / min for 5min to mix evenly, and store at 5°C for later use;
[0046] (4) Add 85ml of the CpG ODN solution obtained in step (1) to the solution in step (3), then stir with a stirrer at 650rpm / min for 5min to emulsify and mix evenly, and place at room temperature for 25min.
[0047] Collect the mixed solution, which i...
Embodiment 3
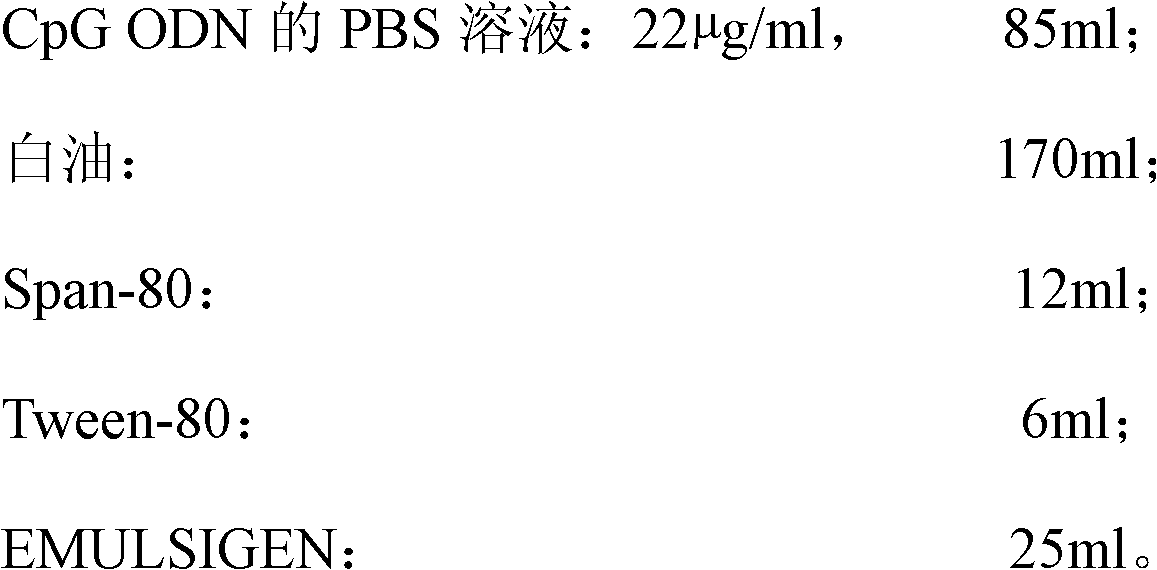
[0049] Prepare raw materials according to the following ratios:
[0050]
[0051] The PBS composition is: by weight percentage, 0.8% NaCl, 0.02% KCl, 0.014% Na 2 HPO 4 , 0.02% KH 2 PO 4 Soluble in water; damp heat autoclave: 118°C, 20min.
[0052] (1) After CpG ODN was synthesized by TAKARA Company, it was prepared with sterile PBS solution to 25 μg / ml, and stored at -20°C for later use;
[0053] (2) Take 172ml of white oil, 11ml of Span-80 and 8ml of Tween-80, mix well with a stirrer at 300rpm / min for 6min, and bathe in water at 68°C for 26min;
[0054] (3) Take 28ml of EMULSIGEN (EM) and the solution obtained in step (2), stir with a stirrer at 280rpm / min for 6min to mix evenly, and store at 6°C for later use;
[0055] (4) Add 90ml of the CpG ODN solution obtained in step (1) into the solution in step (3), then stir with a stirrer at 680rpm / min for 5.5min to emulsify and mix evenly, and place at room temperature for 24min;
[0056] Collect the mixed solution to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com