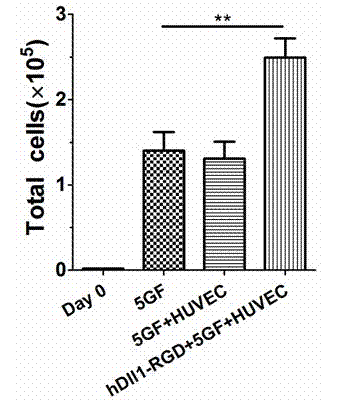
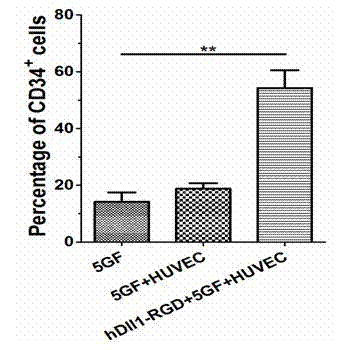
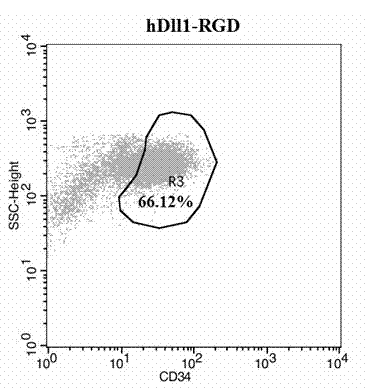
Expansion system in vitro for hematopoietic stem/progenitor cell
An in vitro expansion and hematopoietic stem technology, applied in the direction of blood/immune system cells, animal cells, vertebrate cells, etc., can solve unseen problems and achieve broad clinical application prospects and good hematopoietic reconstruction ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0031] (1) Experimental materials
[0032] (1) Human umbilical vein endothelial cells were cultured as follows
[0033] (2) Umbilical cord blood was obtained from the Department of Obstetrics and Gynecology, Tangdu Hospital, Fourth Military Medical University
[0034] (3) Reagents:
[0035] M199, fetal bovine serum: Gibco
[0036] StemSpan™ serum-free medium, MethoCult™ GF H4434: Stemcell
[0037] Endothelial Cell Growth Supplement (ECGS): Sigma
[0038] Bovine Serum Albumin (BSA): Sigma
[0039] Type II collagenase: Sigma
[0040] Mitomycin C: Sigma
[0041] CD34 MicroBead Kit: Miltenyi Company
[0042] Separation buffer: PBS+2mM EDTA+0.5% BSA
[0043] Lymphocyte Separation Medium: TBD Company
[0044] PBSA: PBS + 2mM EDTA
[0045] SCF (stem cell factor): recombinant human SCF, Peprotech
[0046] TPO (thrombopoietin): recombinant human TPO, Peprotech
[0047] FL (flt3 ligand): recombinant human FL, Peprotech
[0048] IL-6 (interleukin-6): recombinant human IL-6, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com