Positive electrode active material for non-aqueous electrolyte secondary battery, process for production of same, and non-aqueous electrolyte secondary battery produced using same
A cathode active material, non-aqueous electrolyte technology, applied in the field of non-aqueous electrolyte secondary batteries, can solve the problems of reduced battery characteristics, deformation, lack of oxygen, and a large amount of heat, and achieves high mass productivity, inhibition of oxygen deficiency, The effect of suppressing deformation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
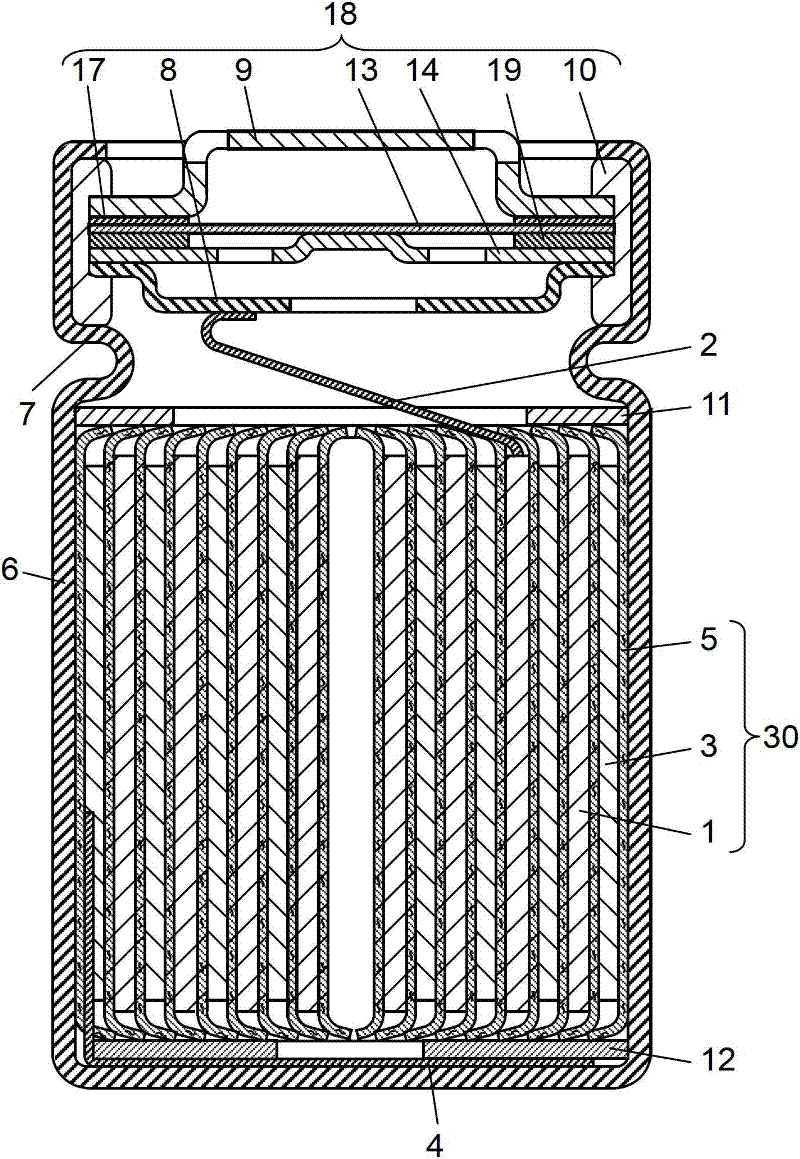
Image
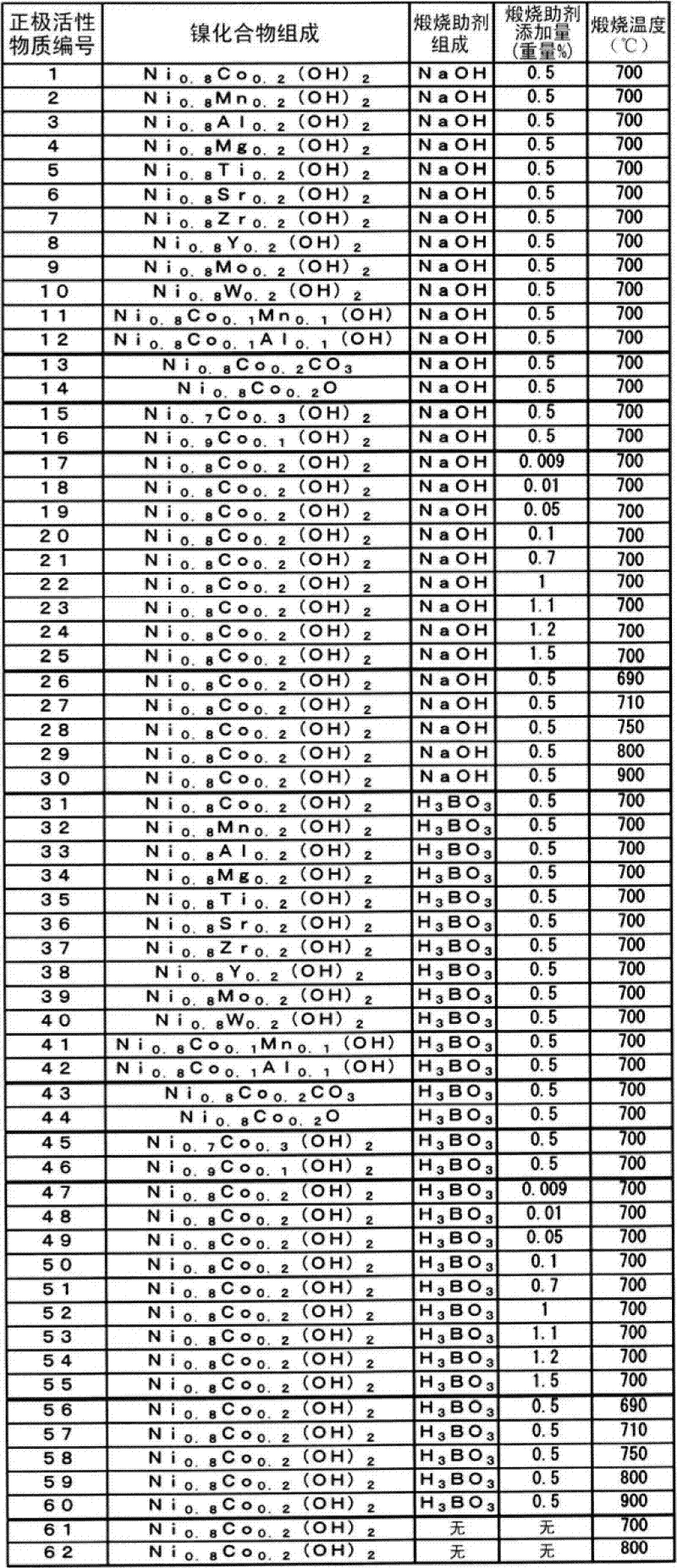
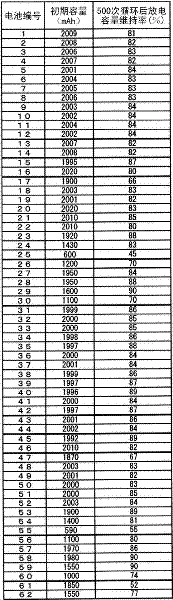
Examples
Embodiment 1
[0057] A nickel-cobalt mixed aqueous solution obtained by adding and dissolving sulfate in a nickel sulfate aqueous solution having an amount corresponding to 60 g / L of metallic nickel so that the molar ratio of Co satisfies Ni:Co=80:20 was prepared. In addition, a 25% by mass sodium hydroxide aqueous solution was used as an alkali agent. The nickel-cobalt mixed aqueous solution whose salt concentration was controlled so that the conductivity reached 120mS / cm was introduced into a precipitation tank with a volume of 100L at a constant rate of 10L / h, and stirred sufficiently while introducing sodium hydroxide solution to generate the Desired composition of nickel hydroxide. The obtained nickel hydroxide was heated at 500° C., thereby preparing nickel oxide (average particle diameter: 10 μm) which is a nickel compound which is one of calcined materials.
[0058] The nickel oxide and lithium hydroxide were mixed so that the atomic ratio of lithium:(nickel+cobalt) reached 1.03:1, a...
Embodiment 2~60
[0076] A cylindrical lithium secondary battery was produced in the same manner as battery No. 1 except that positive electrode active material No. 2 to 60 was used instead of positive electrode active material No. 1 to obtain battery No. 2 to 60.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com