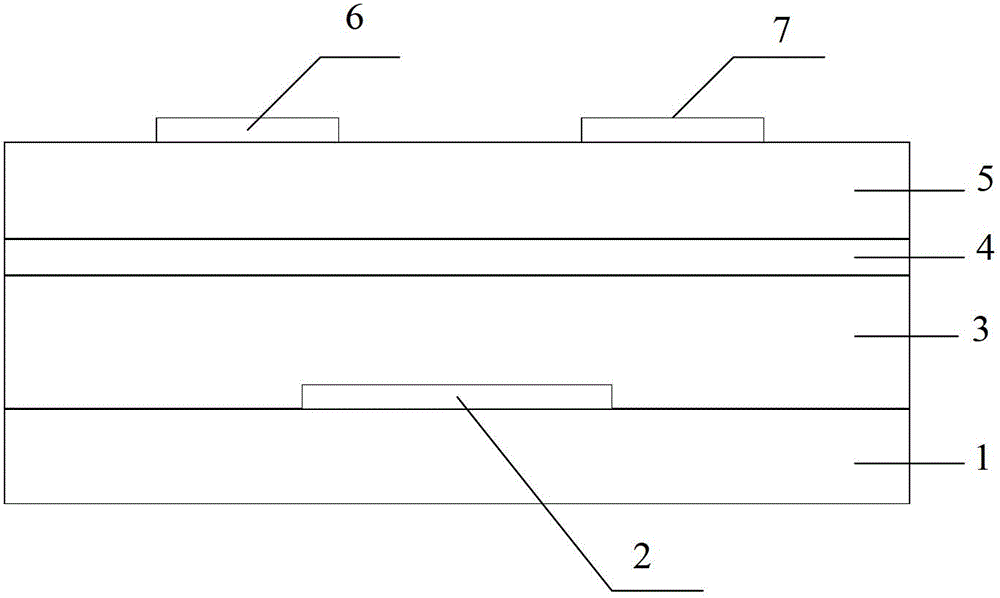
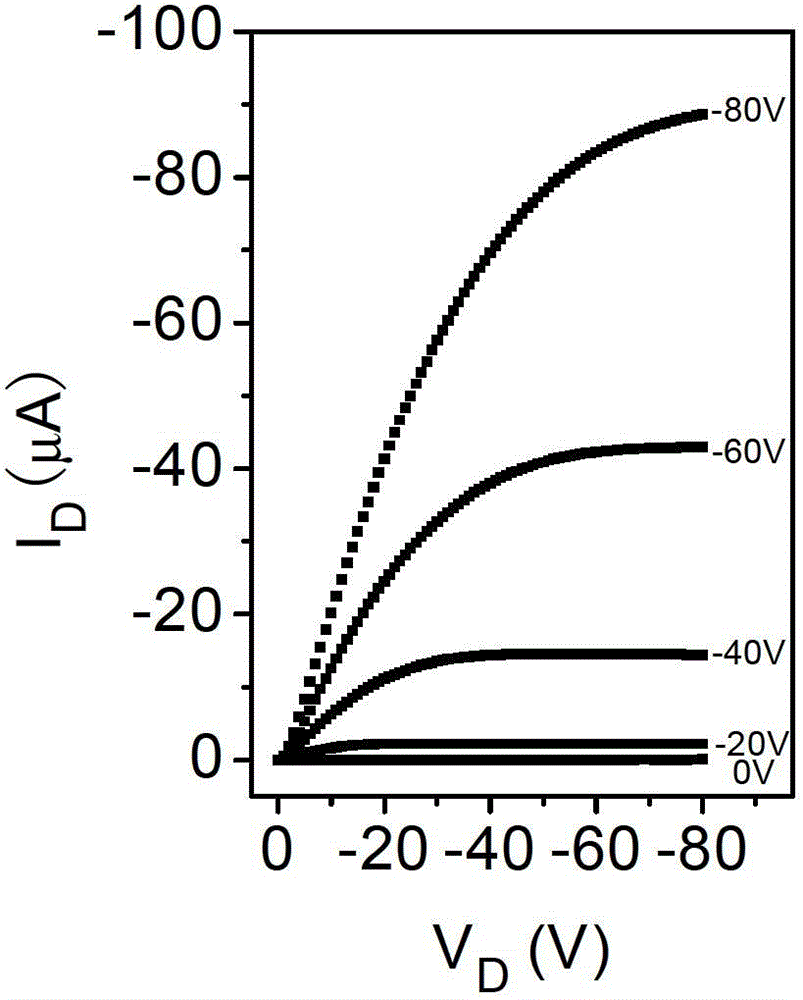
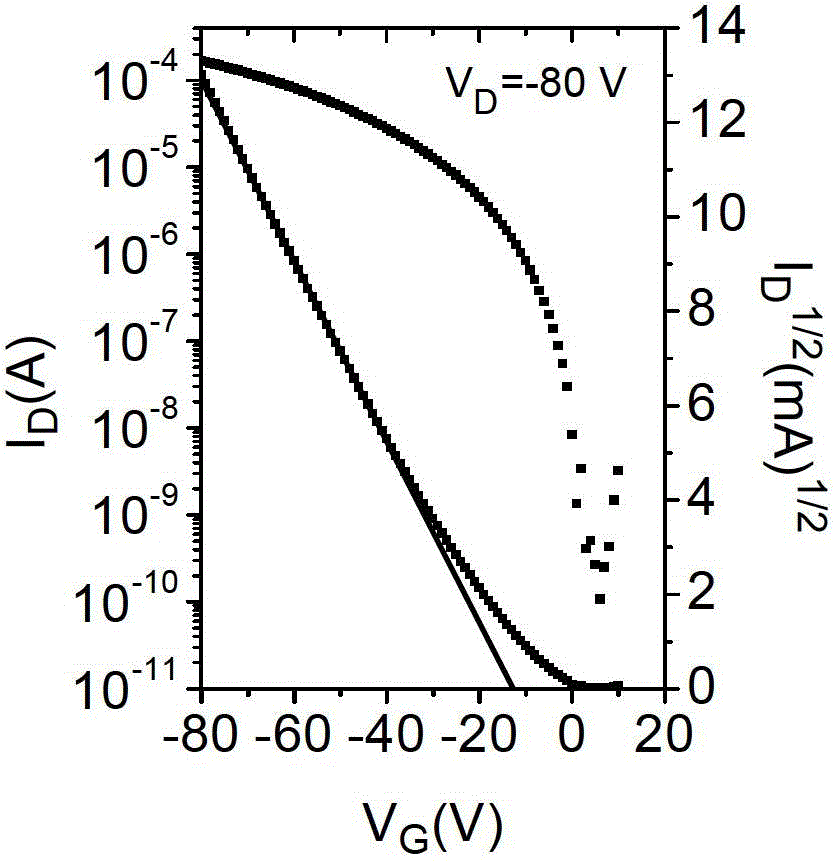
Compound, polymer, polymer semiconductor material and organic thin film transistor
A polymer and semiconductor technology, applied in the fields of semiconductor devices, tin organic compounds, semiconductor/solid-state device manufacturing, etc., can solve problems such as limiting the application of polymer semiconductor materials, achieve good solubility, improve conjugation, and high mobility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085] The invention provides a kind of preparation method of above-mentioned polymer, comprises the following steps:
[0086]Under the protection of an inert gas, the compound with the structure of formula (I) and the polymerized monomer will generate a polymer under the action of a catalyst and an auxiliary ligand; the polymerized monomer has the formula (XII), formula (XIII), formula (XIV ), formula (XV), formula (XVI), formula (XVII), formula (XVIII), formula (XIX) or formula (XX) structure;
[0087]
[0088] Among them, R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , R 12 and R 13 Each independently is an alkyl group, preferably C 12 ~C 30 of alkyl. The R 3 preferably C 12 ~C 30 straight chain alkyl or C 12 ~C 30 branched chain alkyl; the R 4 , R 5 and R 6 each independently preferably C 12 ~C 30 branched chain alkyl.
[0089] In the present invention, when preparing the polymer, under the protection of an inert gas, the compound having ...
Embodiment 1
[0136] Embodiment 1N-dodecyl-2,7-dibromo-3,6-two (methylthio) carbazole synthesis
[0137] Add 9.2mmol dimethyl sulfide to 20mL dichloromethane solution containing 9.2mmol trifluoromethanesulfonic anhydride, stir at -40°C for 15 minutes, then add dropwise 40mL containing 4.4mmol N-dodecyl -2,7-Dibromocarbazole in dichloromethane, stirred at -40°C for 2 hours and then at room temperature for 3 hours, and the solvent was removed in vacuo. Then add 44mL acetonitrile to the system, add 22mmol triethylamine dropwise at 0°C, stir at room temperature for 3 hours, remove the solvent in vacuo, and purify by silica gel column chromatography, the eluent is a mixed solution of dichloromethane and petroleum ether, dichloromethane The volume ratio of methane to petroleum ether was 1:4, and finally a solid, N-dodecyl-2,7-dibromo-3,6-bis(methylthio)carbazole, was obtained with a yield of 53.0%. The structural characterization data are as follows: 1 H NMR (300MHz, CDCl3): δ (ppm) 7.92 (s, 2H...
Embodiment 2
[0138] Example 2 Preparation of N-dodecyl-2,7-bis(trimethylsiliethynyl)-3,6-bis(methylthio)carbazole
[0139] Under argon atmosphere, add 2.1mmol N-dodecyl-2,7-dibromo-3,6-bis(methylthio)carbazole, 72mg bis(triphenylphosphine)palladium dichloride (Pd (PPh 3 ) 2 Cl 2 ), 39mg of cuprous iodide (CuI), and 20mL of triethylamine were slowly added dropwise with 4.53mmol of trimethylsilylacetylene, stirred at room temperature for 28 hours, washed with ethyl acetate, collected the organic phase, and washed with saturated ammonium chloride solution Wash the organic phase twice with saturated saline, dry over anhydrous magnesium sulfate, remove the solvent, and purify with silica gel column chromatography. The eluent is a mixed solution of dichloromethane and petroleum ether. The volume ratio of dichloromethane to petroleum ether is The ratio is 1:4, and finally a solid is obtained, that is, N-dodecyl-2,7-bis(trimethylsilylethynyl-3,6-bis(methylthio)carbazole, the yield is 70.0%, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| carrier mobility | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com