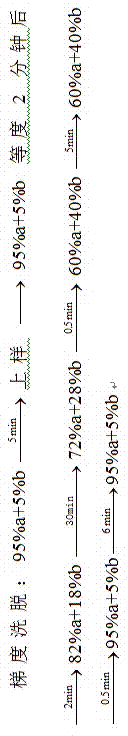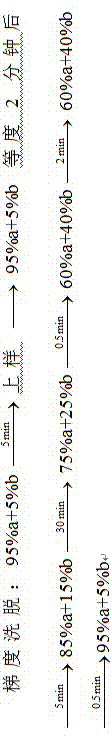Method for preparing eptifibatide
A technology for the preparation of eptifibatide and solid phase, applied to the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of no large-scale production, low yield of purification process, etc., and reduce exposure to alkali The probability of increasing the yield and avoiding the effect of oxidation impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
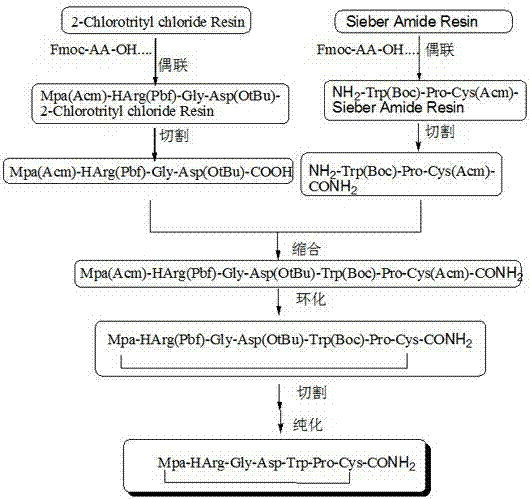
[0059] Example 1: Preparation of Fmoc-Asp(OtBu)-CTC Resin with a degree of substitution of 0.6mmol / g
[0060] Weigh 300 g of 2-CTC resin with a substitution degree of 1.0 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then take 86.31 g of Fmoc-Asp(OtBu)-OH for use DMF was dissolved, activated by adding 89.38mL DIPEA in an ice-water bath, then added to the above-mentioned reaction column equipped with resin, and reacted for 2 hours at a controlled reaction temperature of -5°C. Add 260mL of anhydrous methanol to block for 30min. Wash with DMF for 3 times, DCM for 3 times, shrink and dry with methanol to obtain Fmoc-Asp(OtBu)-CTC resin, and the detected substitution degree is 0.615mmol / g.
Embodiment 2
[0061] Example 2: Preparation of Fmoc-Asp(OtBu)-CTC Resin with a degree of substitution of 1.0mmol / g
[0062] Weigh 300 g of 2-CTC resin with a substitution degree of 1.2 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then take 147.96 g of Fmoc-Asp(OtBu)-OH for use DMF was dissolved, activated by adding 153.23mL DIPEA under an ice-water bath, then added to the above-mentioned reaction column equipped with resin, and reacted for 2 hours at a controlled reaction temperature of -5°C. Add 260mL of anhydrous methanol to block for 30min. Wash with DMF for 3 times, DCM for 3 times, shrink and dry with methanol to obtain Fmoc-Asp(OtBu)-CTC resin, the detection degree of substitution is 0.975mmol / g.
Embodiment 3
[0063] Example 3: Preparation of Fmoc-Asp(OtBu)-CTC Resin with a degree of substitution of 0.8mmol / g
[0064]Weigh 300g of 2-CTC resin with a substitution degree of 1.0mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, take 111.09g of Fmoc-Asp(OtBu)-OH for use DMF was dissolved, activated by adding 114.93mL DIPEA under an ice-water bath, then added to the above-mentioned reaction column equipped with resin, and reacted for 2 hours at a controlled reaction temperature of -5°C. Add 260mL of anhydrous methanol to block for 30min. Wash with DMF for 3 times, DCM for 3 times, shrink and dry with methanol to obtain Fmoc-Asp(OtBu)-CTC resin, the detection degree of substitution is 0.775mmol / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com