Straight-chain oligosaccharide library and preparation method thereof
An oligosaccharide and straight-chain technology, applied in the field of straight-chain oligosaccharide library and preparation thereof, can solve the problems of time-consuming and laborious, complex oligosaccharide synthesis method, etc., and achieve the effects of low price, few synthesis steps, and easy availability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
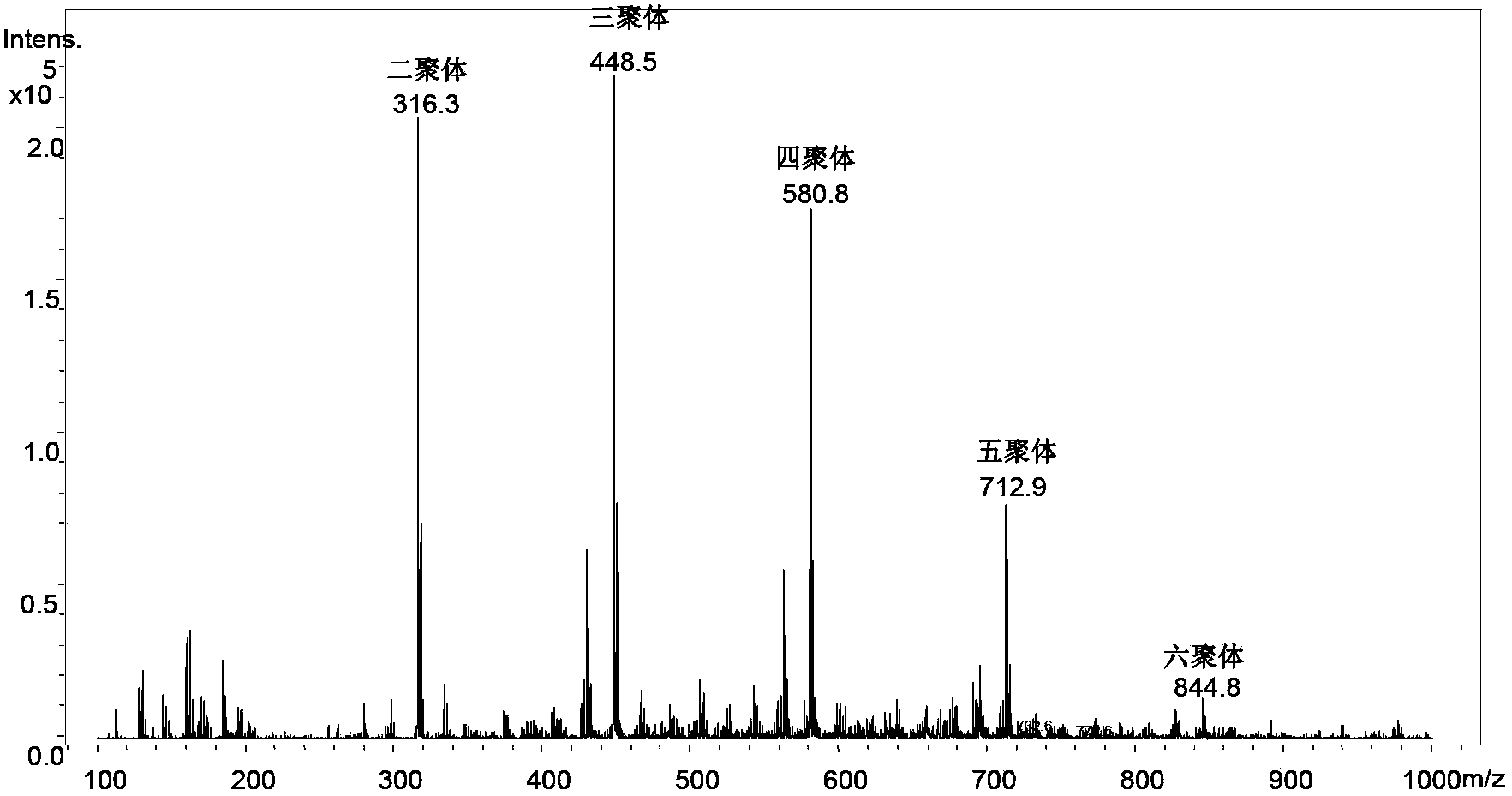
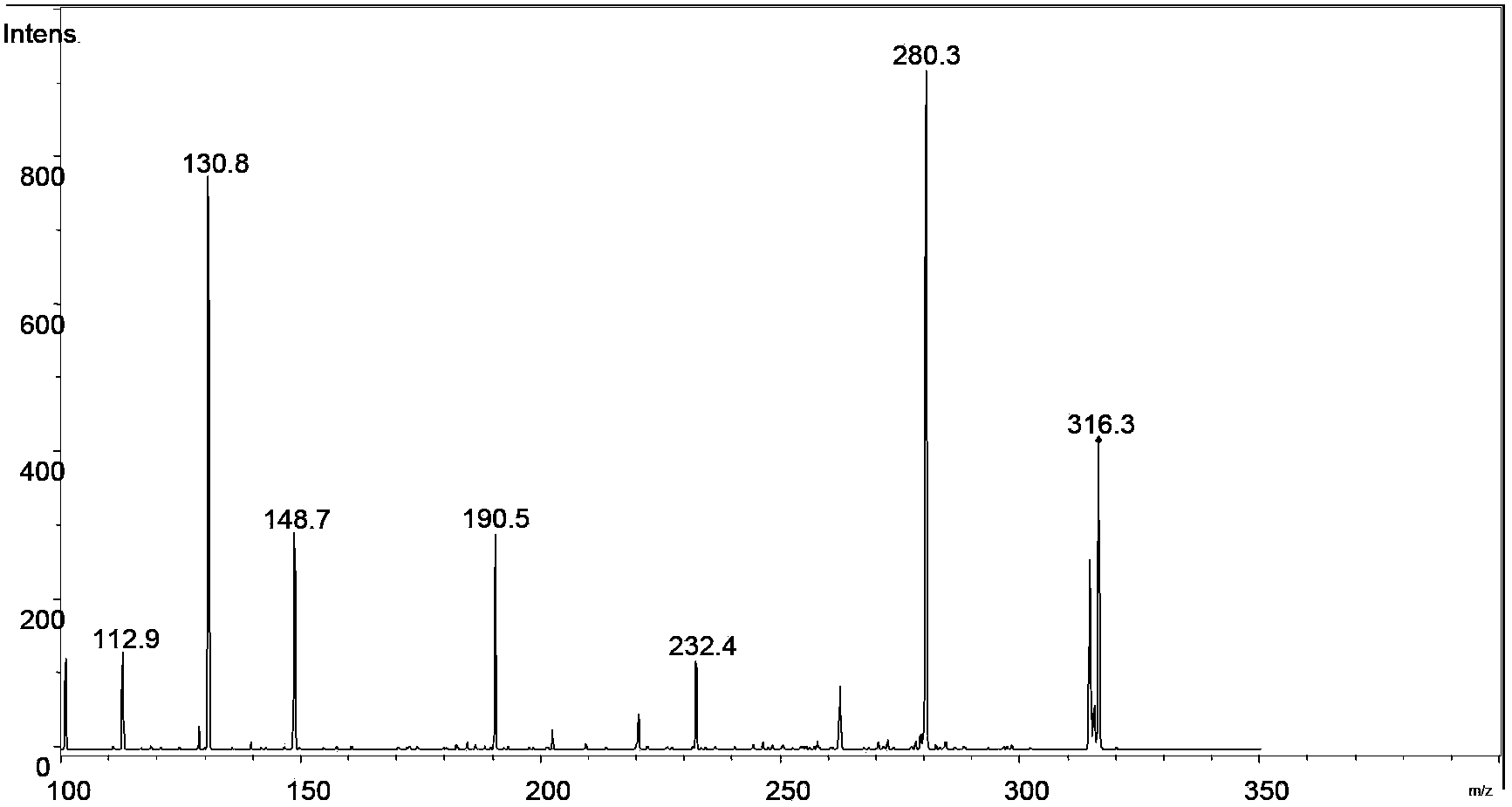
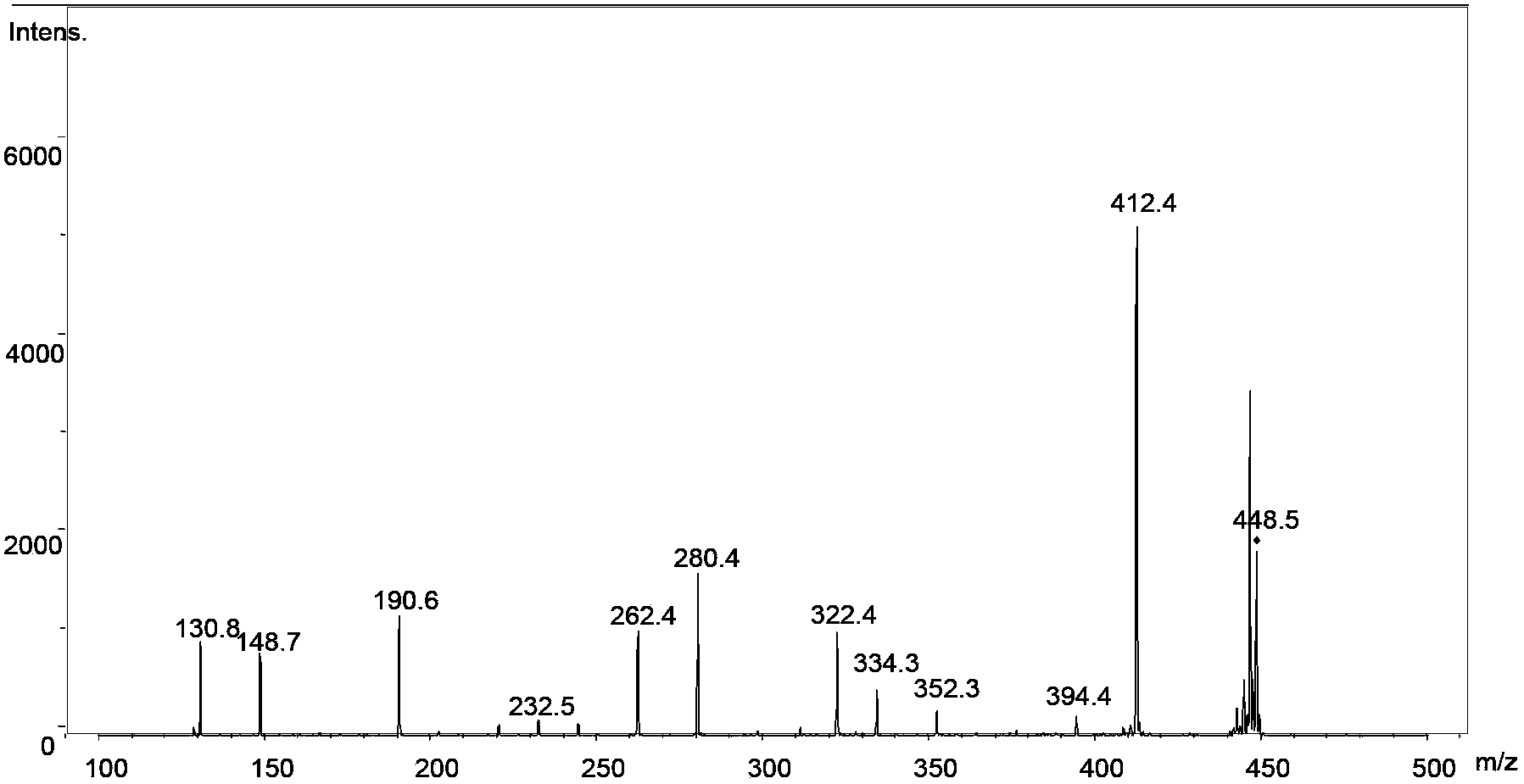
[0051] Add triphenylphosphine and halohydrocarbon with a molar ratio of 1:1.2 into the container, dissolve in 50mL organic solvent under nitrogen protection, stir for 6h to generate dichlorotriphenylphosphine and then add 1 equivalent of . The D-ribose was dehydrated in a vacuum, and stirred for 72 hours in an ice-water bath. Add 30mL of water after distilling off THF under reduced pressure, stir in an ice-water bath for 1 hour, and then filter under reduced pressure. Oligosaccharide library of ribose. Using ESI-MS n Identify the generation of the oligosaccharide library, and use multi-stage mass spectrometry to identify the structure of each oligosaccharide. The relevant mass spectrometry results are as follows: Figure 1~6 shown. The structures of various oligosaccharide products in the library and the corresponding mass-to-charge ratios detected by mass spectrometry are shown below:
[0052]
[0053] Ribose dimer [Disaccharide+Cl] - :317.06m / z
[0054]
[0055] Ri...
Embodiment 2
[0063] Dissolve triphenylphosphine and halogenated hydrocarbons with a molar ratio of 1:1.2 in 50 mL of organic solvent under the protection of nitrogen, stir for 6 hours, and add 1 equivalent of D-xylose under nitrogen atmosphere to generate dihalotriphenylphosphine , and stirred for 72h under ice-water bath conditions. Add 30mL of water after distilling off THF under reduced pressure, stir in an ice-water bath for 1 hour, and then filter under reduced pressure. Oligosaccharide library of xylose. Using ESI-MS n Identify the generation of the oligosaccharide library, and use multi-stage mass spectrometry to identify the structure of each oligosaccharide. The relevant mass spectrometry results are as follows: Figure 7-12 shown. The structures of various oligosaccharide products in the library and the corresponding mass-to-charge ratios detected by mass spectrometry are shown below:
[0064]
[0065] Xylose dimer [Disaccharide+Cl] - :317.06m / z
[0066]
[0067] Xylo...
Embodiment 3
[0075] Dissolve triphenylphosphine and halogenated hydrocarbons with a molar ratio of 1:1.2 in 50 mL of organic solvent under nitrogen protection, stir for 6 h, and add 1 equivalent of D-glucose under nitrogen atmosphere after generating dihalotriphenylphosphine. It was stirred for 72h under the condition of ice-water bath. Add 30mL of water after distilling off THF under reduced pressure, stir in an ice-water bath for 1 hour, and then filter under reduced pressure. Oligosaccharide library of glucose. Using ESI-MS n Identify the generation of the oligosaccharide library, and use multi-stage mass spectrometry to identify the structure of each oligosaccharide. The relevant mass spectrometry results are as follows: Figure 13~17 shown. The structures of various oligosaccharide products in the library and the corresponding mass-to-charge ratios detected by mass spectrometry are shown below:
[0076]
[0077] Glucose dimer [Disaccharide+Cl] - :377.09m / z
[0078]
[0079...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com