Method for preparing 7alpha, 15alpha-dihydroxy androstenone by pre-inducing substrate
A technology of hydroxyandrostenone and substrate, which is applied in the field of bioengineering and can solve the problems of limited transformation ability and a large number of toxic reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Place the inoculated slant in a constant temperature incubator at 30°C and culture it for 3 days to obtain mature spores. Inoculate the spores into a 500mL shaker flask containing 100mL seed medium and shake at 28°C and 220rpm. Cultivate on the bed for 4 days to obtain seed culture solution suitable for inoculation.
[0021] (2) Put the seed culture solution into a 250mL shake flask containing 30mL of fermentation medium at an inoculum amount of 10%, and continue culturing for 24h under the same conditions to obtain a cell culture solution.
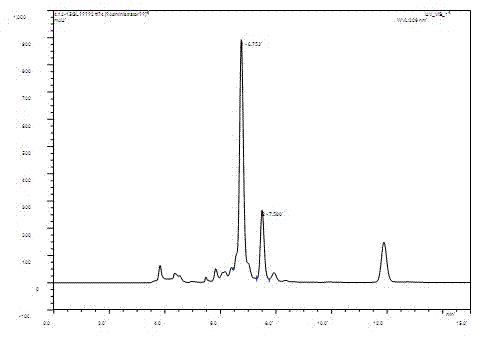
[0022] (3) Accurately weigh 8 g / L of the substrate and put it into the bacterial cell fermentation liquid obtained in step (2), transform it under the same conditions for 60 hours and put it into a bottle. The extracted product was analyzed by HPLC, and the conversion rate was 83.79%.
Embodiment 2
[0024] (1) Slope culture, seed culture and fermentation culture are the same as in Example 1.
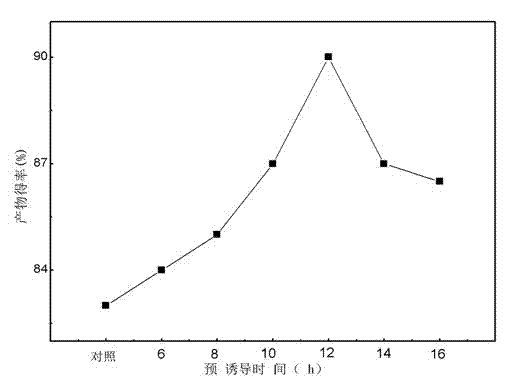
[0025] (2) Put the seed culture solution into a 250mL shake flask containing 30mL fermentation medium at an inoculum amount of 10%, and cultivate it for 12 hours under the culture conditions of 28°C and 220rpm, and then accurately weigh 2g / L (substrate weight / volume of fermentation broth) the substrate was put into the cell fluid and continued to cultivate for 12h.
[0026] (3) Accurately weigh 8 g / L of the substrate dehydroepiandrosterone and add it to the bacterial cell culture solution obtained in step (2), so that the final concentration of the substrate is 8 g / L, continue the transformation for 48 hours and put it in the bottle. Take 1mL of fermentation broth to detect, and the conversion rate is 89.95%.
Embodiment 3
[0028] (1) Slope culture, seed culture and fermentation culture are the same as in Example 1.
[0029] (2) Put 10% inoculum of the seed culture solution into a 250mL shake flask containing 30mL fermentation medium, cultivate for 8h, culture conditions are 28°C, 220rpm, and then accurately weigh 2g / L (substrate weight / Fermentation broth volume) the substrate was put into the cell fluid to continue culturing for 16 hours.
[0030] (3) Accurately weigh 8 g / L of the substrate dehydroepiandrosterone and add it to the bacterial cell culture solution obtained in step (2), so that the final concentration of the substrate is 8 g / L, continue the transformation for 48 hours and put it in the bottle. Take 1mL of fermentation broth to detect, and the conversion rate is 86.95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com