Inhibitors of receptor tyrosine kinases (rtk) and methods of use thereof
A technology of tyrosine kinase and amino acid, which is applied to the inhibitor of receptor tyrosine kinase (RTK) and its application field, can solve the problem of not knowing what the structural basis of receptor tyrosine kinase is.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0244] Preparation of polyclonal antibody of the present invention
[0245] Polyclonal antibodies of the invention can be produced by a variety of techniques well known in the art. Polyclonal antibodies are derived from different B cell lines and therefore recognize multiple epitopes on the same antigen. Polyclonal antibodies are typically produced by immunizing a suitable mammal with an antigen of interest (eg, the asymmetric contact interface of an RTK). Animals frequently used to generate polyclonal antibodies are chickens, goats, guinea pigs, hamsters, horses, mice, rats, sheep and most commonly rabbits. Standard methods for producing polyclonal antibodies are widely known in the art and can be combined with methods of the present invention (e.g. research.cm.utexas.edu / bkitto / Kittolabpage / Protocols / Immunology / PAb.html; U.S. Patents 4,719,290, 6,335,163, 5,789,208, 2,520,076, 2,543,215, and 3,597,409, the entire contents of which are incorporated herein by reference). ...
Embodiment 1
[0343] Example 1: Asymmetric dimerization interface during autophosphorylation of FGFR1.
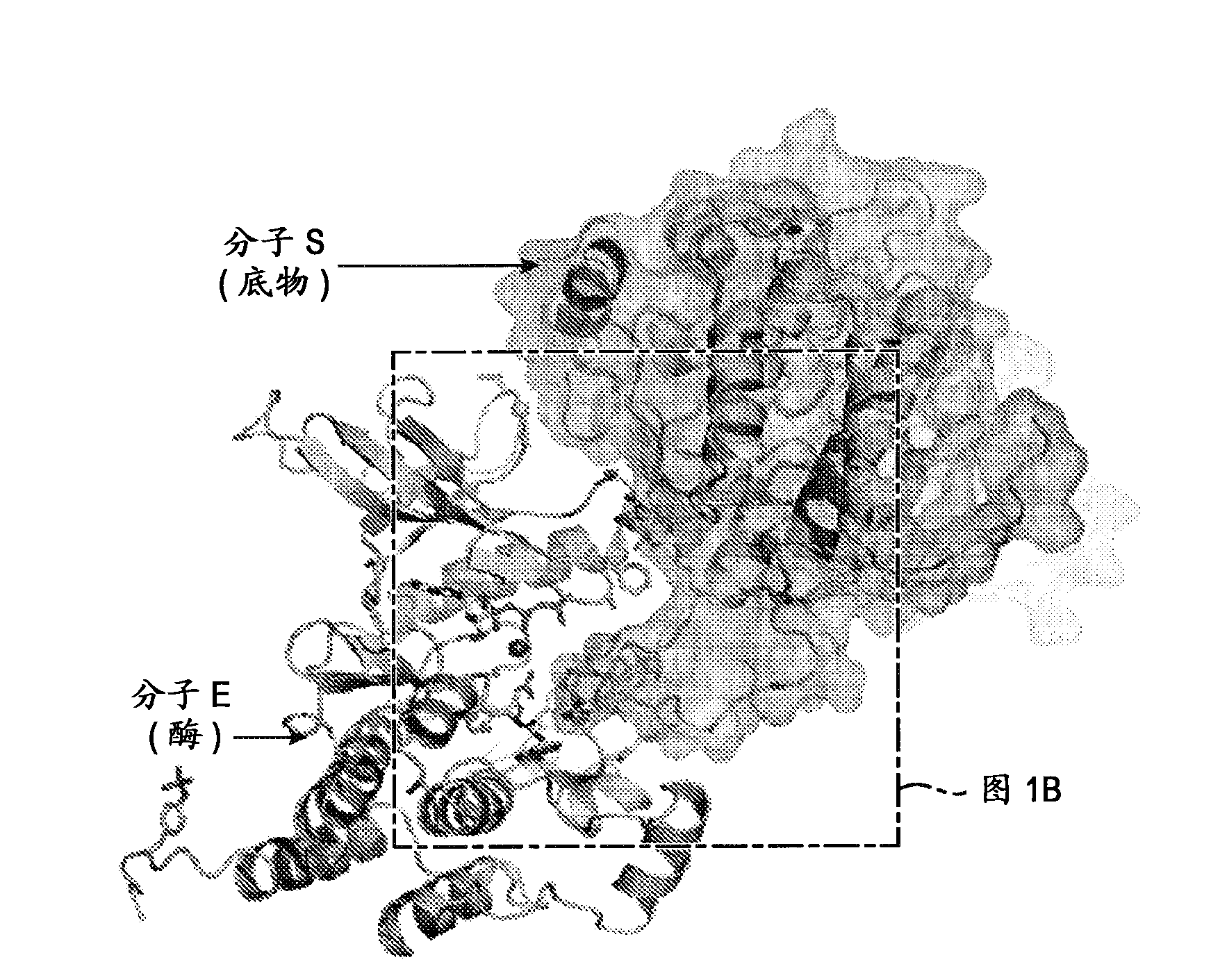
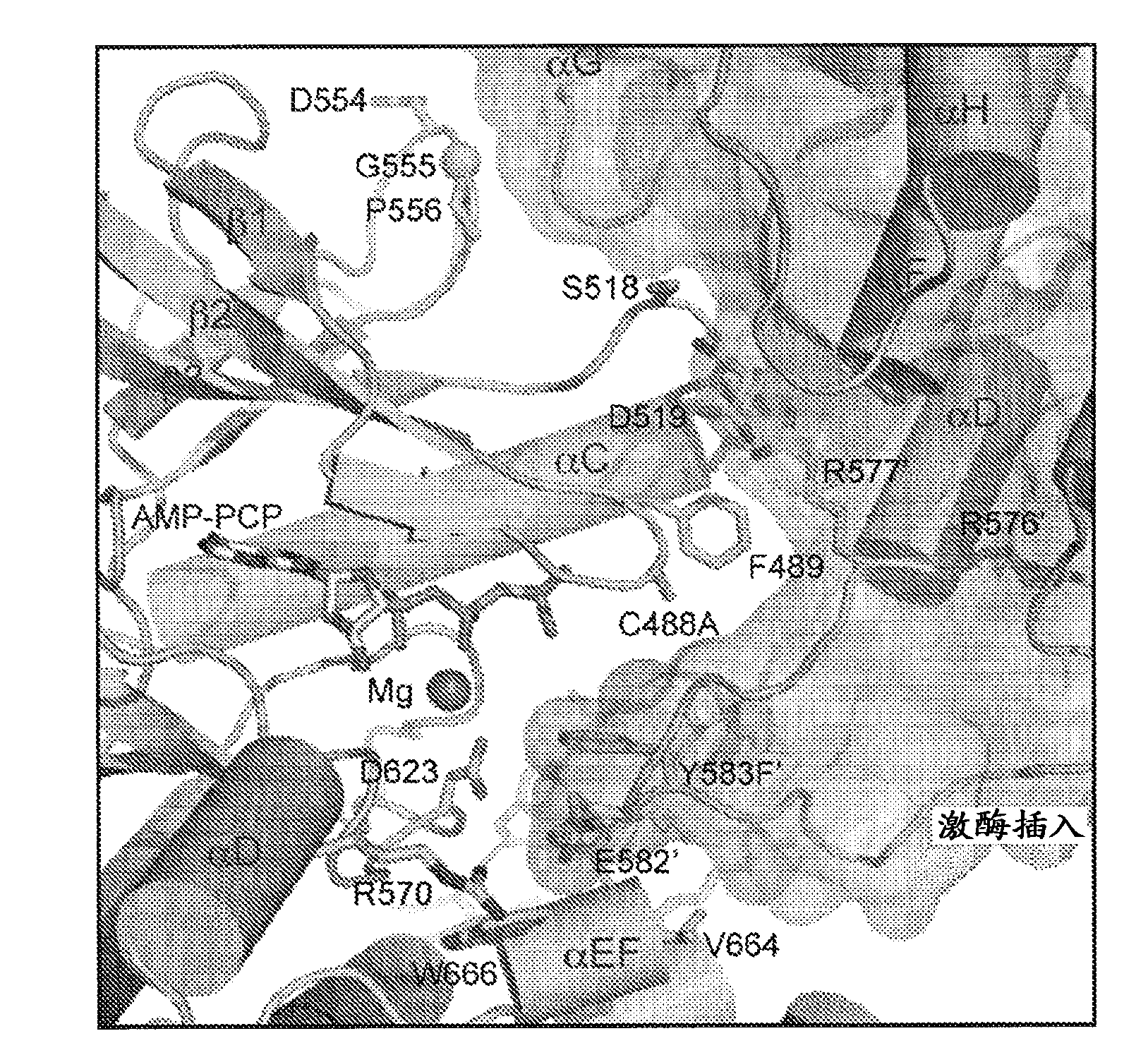
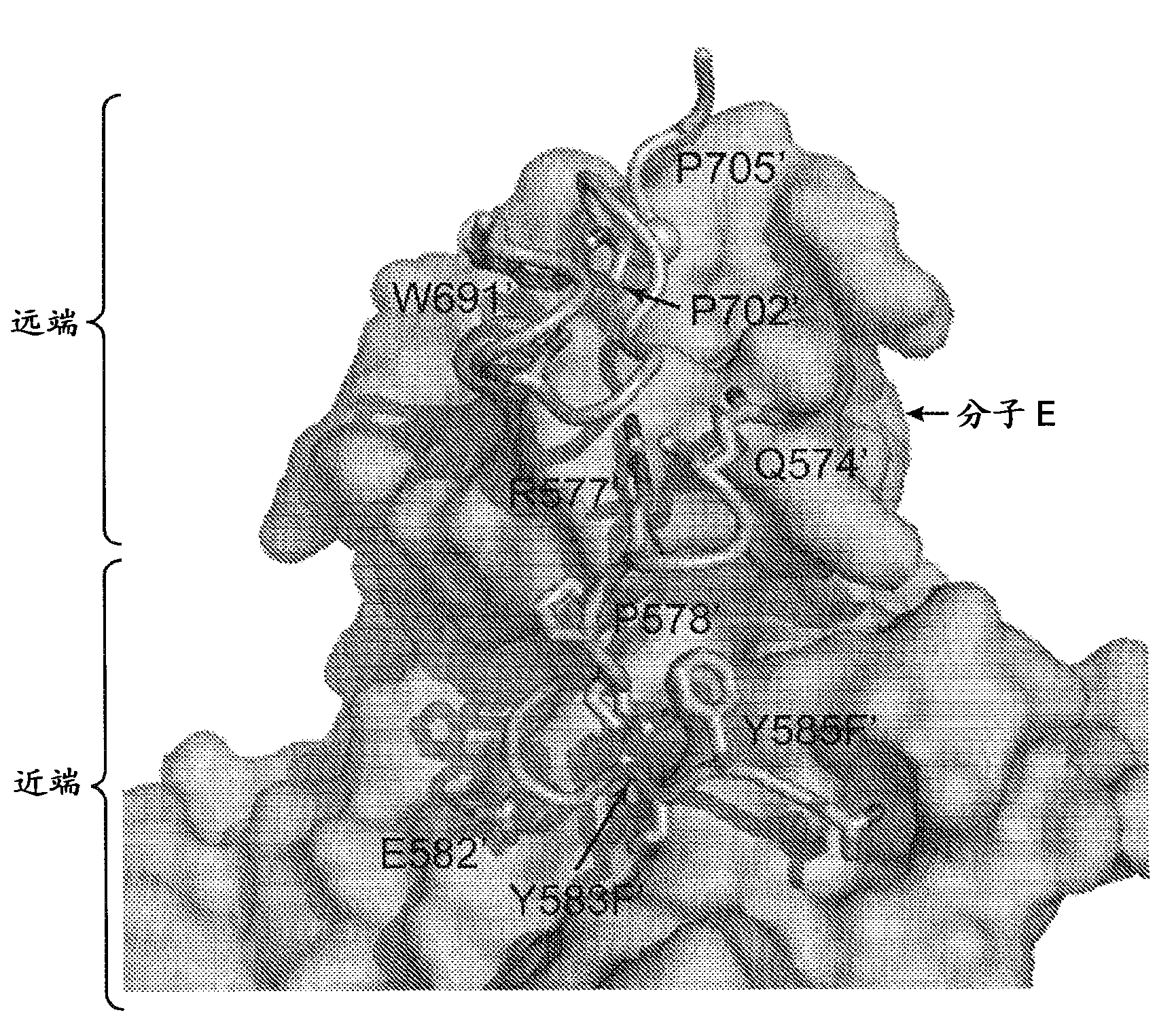
[0344] Structure of activated FGFR1 kinase in complex with a phospholipase Cγ (PLCγ) fragment (Bae et al. (2009) Cell, 138(3):512-524) shows two symmetrically related activated kinase domains forming an asymmetric dimerization body showing in vivo trans-autophosphorylation of Y583 in the kinase insert region ( Figure 1A and Figure 1B ). The asymmetric arrangement of the two kinase molecules is formed between the activation fragment, the tip of the nucleotide-binding loop, the β3-αC loop, the β4-β5 loop, and the N-terminal region of the αC helix in the kinase molecule used as enzyme (E). The interface formed between the kinase insertion and the residues between the C-lobe helix αF-αG of the second kinase molecule used as the substrate (S) is mediated by ( Figure 1C and Figure 1D ). Importantly, R577 (a residue close to the kinase insertion region of the substrate molecule) contrib...
Embodiment 2
[0347] Example 2: In vitro tyrosine kinase activity of the R577E FGFR1 mutant.
[0348] To investigate the in vitro effects of the R577E mutant (FGFR1-RE), autophosphorylation assays were performed with wt-FGFR1 and the kinase domain of FGFR1-RE. with ATP and Mg at room temperature 2+ Purified FGFR1 kinase domains were incubated and monitored at various times by stopping the trans-autophosphorylation reaction with EDTA and running all samples on non-reducing native gels ( Figure 3A and Figure 3B ). Response profiles of wt-FGFR1 and FGFR1-RE on pristine gels clearly show that the trans autophosphorylation and reverse dephosphorylation responses of FGFR1-RE are significantly delayed compared to the wt-FGFR1 kinase domain. Trans autophosphorylation of the wt-FGFR1 kinase domain occurs within 10 min, reaching a fully phosphorylated state, which then undergoes reverse dephosphorylation. This is in contrast to FGFR1-RE, which becomes fully phosphorylated within 30 minutes and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com