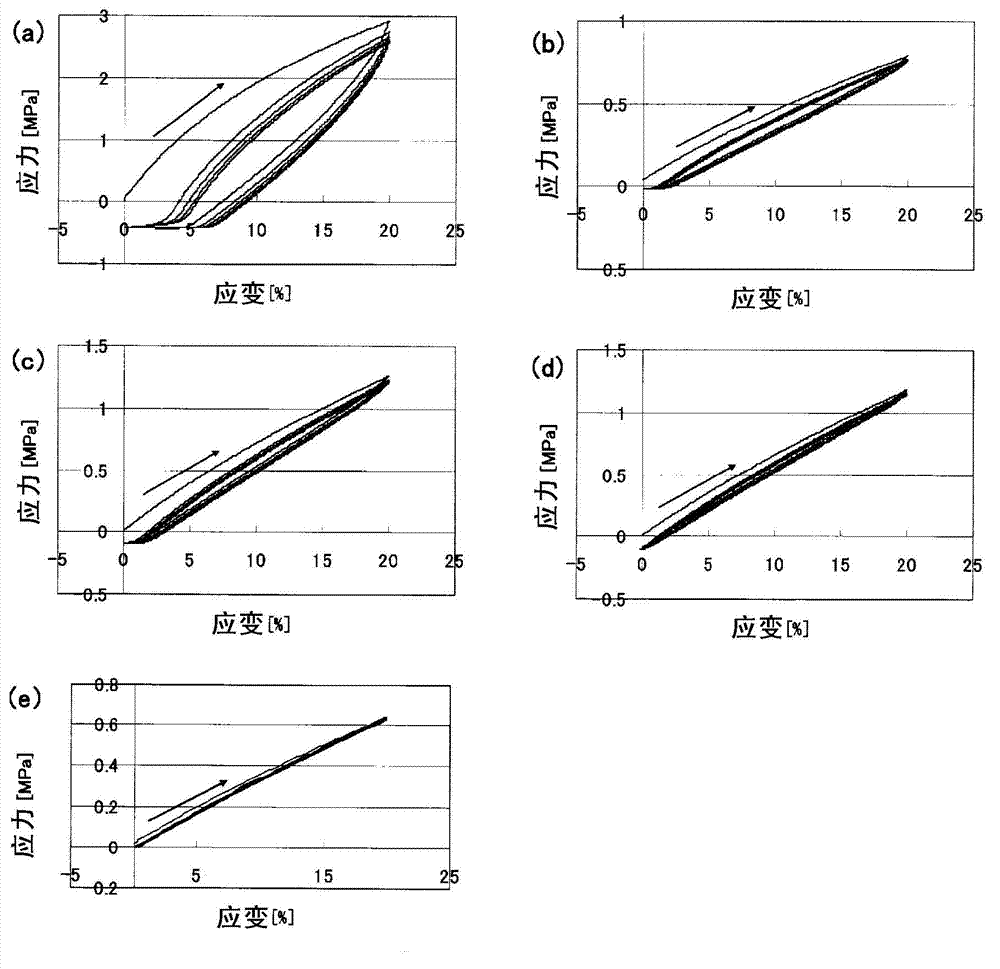
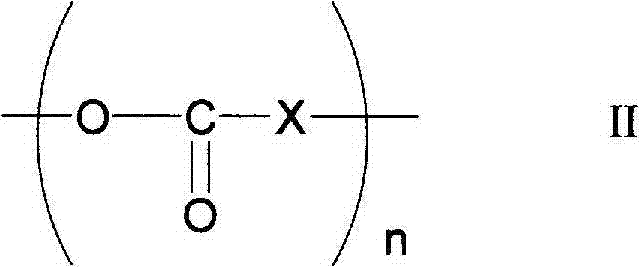
Photocrosslinkable polyrotaxane, composition comprising the photocrosslinkable polyrotaxane, crosslinked body derived from the composition, and methods for producing same
A technology of photocrosslinking and polyrotaxane, applied in the field of crosslinkers, can solve the problem of low degree of modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment )
[0223] The present invention will be described in further detail below based on the examples, but the present invention is not limited by the examples.
Embodiment 1
[0225] According to the method described in WO2005-080469 (wherein, the content of this document is incorporated in this specification as a reference), the linear molecule: polyethylene glycol (average molecular weight: 35,000), the cyclic molecule: α-cyclogluter fine (hereinafter referred to as "cyclodextrin" as "CD"), capping group: a compound obtained by further hydroxypropylation of polyrotaxane formed by adamantine group (hereinafter referred to as hydroxypropylated polyrotaxane "HAPR35") (α-CD inclusion ratio: 25%; hydroxypropyl modification ratio: 50%; theoretical hydroxyl group amount: 9.7 mmol / g; weight average molecular weight Mw based on gel permeation chromatography (GPC): 150000). The measurement of molecular weight and molecular weight distribution was carried out with a TOSOHHLC-8220GPC device. Chromatographic column: TSK guard column Super AW-H and TSKgel Super AWM-H (two connected), eluent: dimethyl sulfoxide / 0.01MLiBr, column temperature: 50°C, flow rate: 0.5...
Embodiment 2
[0232] Except that 21.5 g of ε-caprolactone and 1.0 g of α-methyl-γ-butyrolactone were used instead of 22.5 g of ε-caprolactone in of Example 1, the same operation was carried out as in Example 1 to produce a modified polymer Rotaxane A-2. As measured by GPC, the weight average molecular weight Mw of the obtained polyrotaxane was 580,000.
[0233] The degree of polymerization of the copolymer of ε-caprolactone and α-methyl-γ-butyrolactone was investigated by the amount of theoretical hydroxyl groups of the raw material HAPR35 and the monomer consumption of gas chromatography (GC) (almost 100%). As a result, it was found that, The degree of polymerization of the obtained modified polyrotaxane A-2 was 4.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elastic modulus | aaaaa | aaaaa |
| hysteresis loss | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com