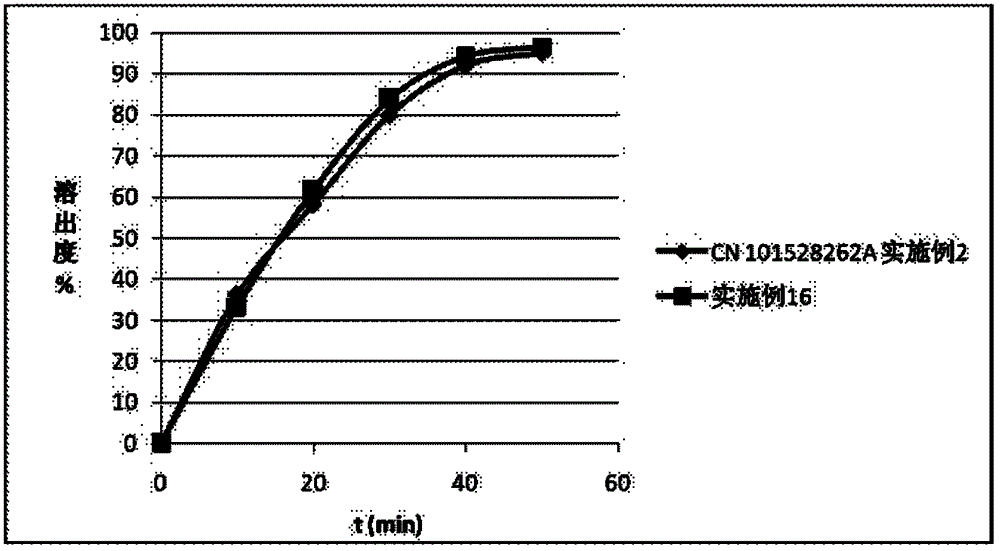
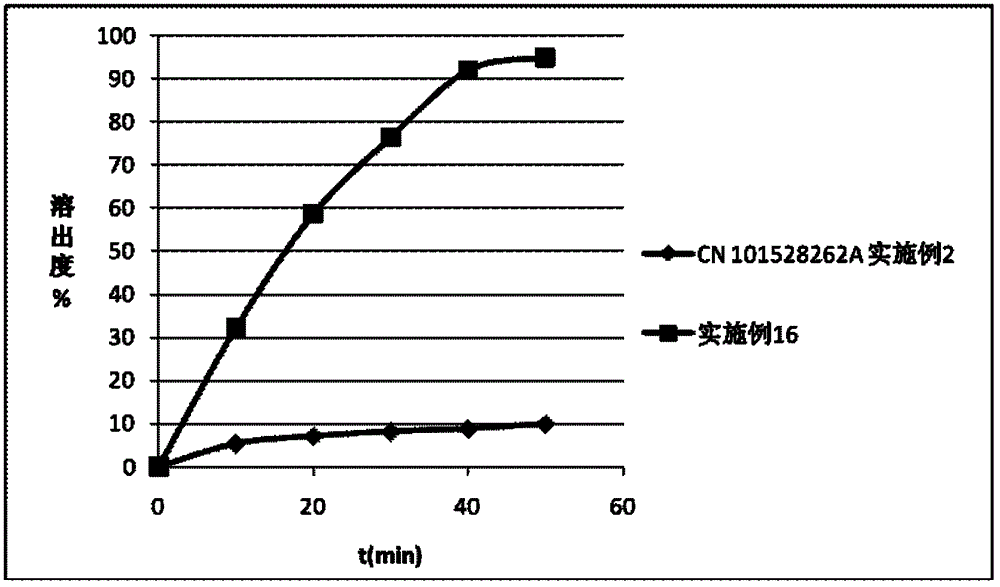
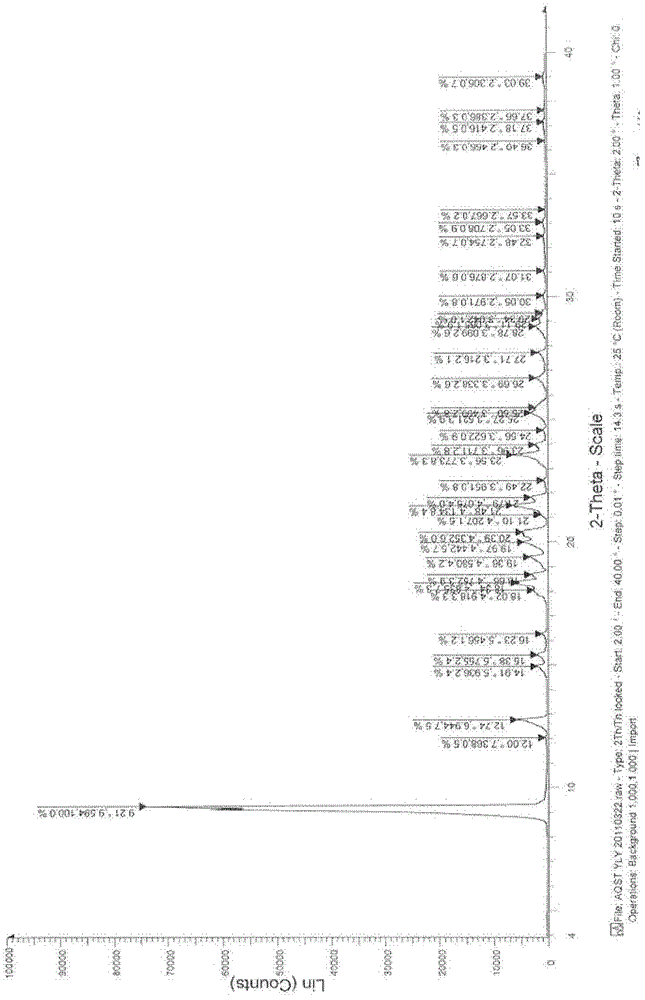
Azilsartan solid dispersion and preparation method and medicinal composition thereof
A technology of solid dispersion and composition, which is applied in the direction of drug combination, non-active ingredient medical preparations, pharmaceutical formulations, etc., can solve the problems such as the absorption and poor bioavailability of pharmaceutical active ingredients, and reduce pH dependence. , Improve solubility and good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 5g of azilsartan and 10g of povidone PVP-K30, add 400mL of methanol and stir until dissolved, transfer them to a vacuum drying oven, keep at 40°C, dry under reduced pressure for 24h, then pulverize, pass through a 60-mesh sieve, That is, azilsartan solid dispersion was obtained.
Embodiment 2
[0033] Weigh 5g of azilsartan and 15g of povidone PVP-K30, add 400mL of methanol and stir until dissolved, transfer them to a vacuum drying oven, keep at 40°C, dry under reduced pressure for 24 hours, then pulverize, pass through a 60-mesh sieve, That is, azilsartan solid dispersion was obtained.
Embodiment 3
[0035] Weigh 5g of azilsartan and 17.5g of povidone PVP-K30, add 400mL of methanol and stir until dissolved, transfer them to a vacuum drying oven, keep at 40°C, dry under reduced pressure for 24 hours, then pulverize and pass through a 60-mesh sieve , to obtain azilsartan solid dispersion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com