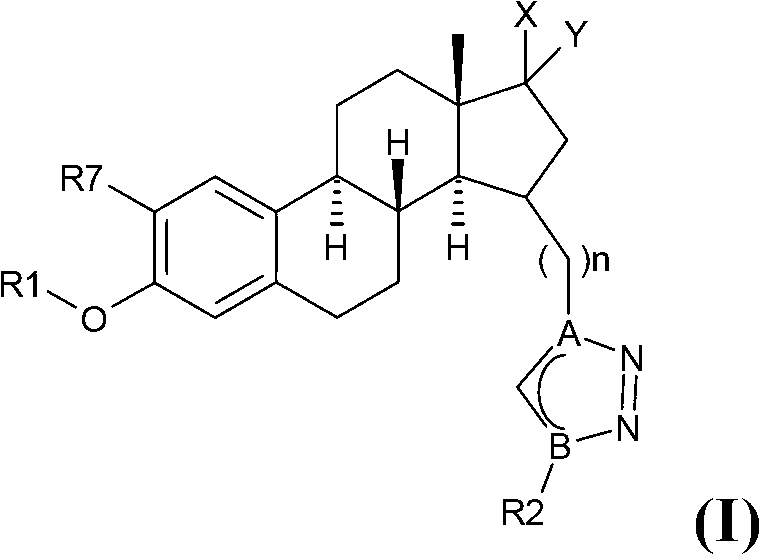
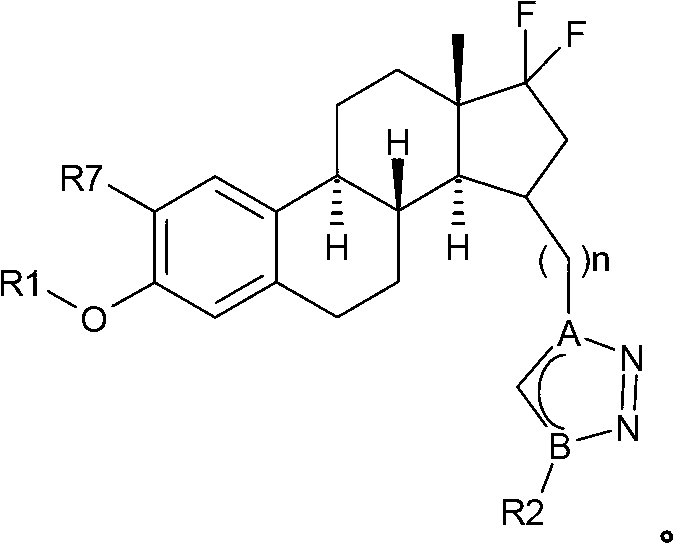
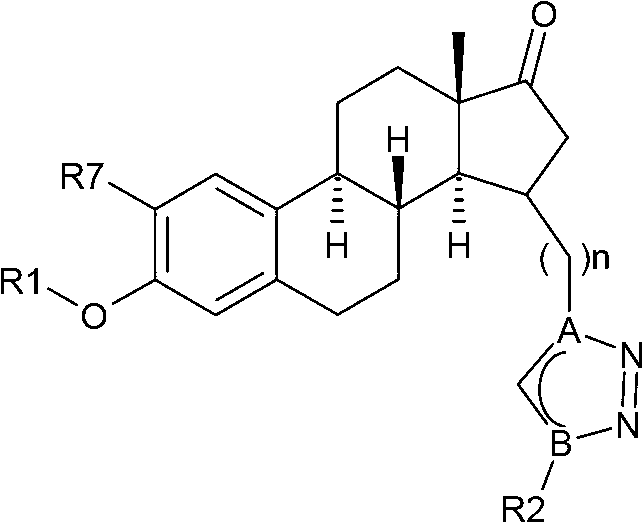
Estratriene derivatives and their uses as 17beta-hydr0xyster0id dehydrogenase inhibitors
A technology of alkyl and nitrile groups, applied in the field of novel estriene-triazole derivatives, can solve the problems such as the inability to reach the level of T castration in men
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0390] Experimental part
[0391] Examples of the preparation of compounds of the invention are provided in the detailed synthetic procedures below. In the compound tables that follow, the synthesis of individual compounds refers to these exemplary preparative procedures.
[0392] Compounds of formula I can be prepared using the reactions and techniques described in this section. The reactions are performed in solvents appropriate to the reagents and materials employed and suitable for the transformations resulting. Meanwhile, in the synthesis method described below, it should be understood that the selection of all proposed reaction conditions is the standard conditions of the reaction, including the choice of solvent, reaction atmosphere, reaction temperature, experiment duration and post-treatment operation, and the above conditions are easily determined by those skilled in the art. Technicians understand. Those skilled in the art of organic synthesis will appreciate tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com