Medicine composition of oral rehydration salt
A technology for rehydration salts and mixtures, applied in the field of medicine, can solve the problems of unqualified uniformity of finished products, agglomeration, and unsuitable dispersion of products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
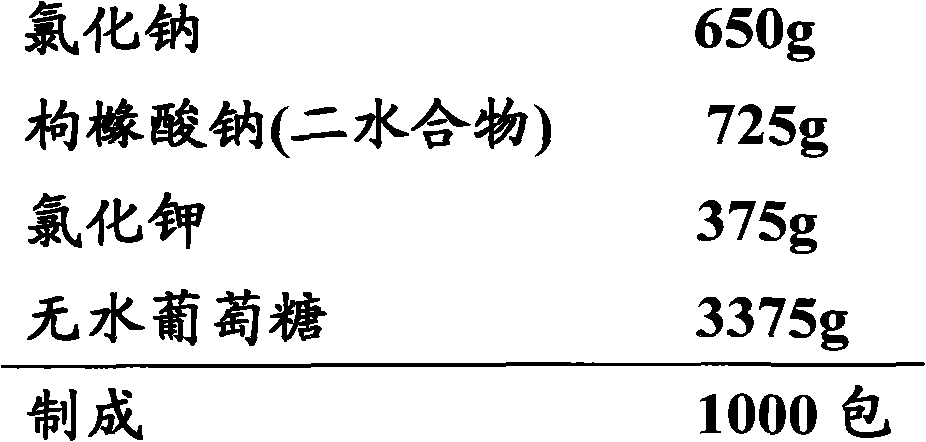
[0160] prescription: 375 parts by weight of potassium chloride, 650 parts by weight of sodium chloride, 725 parts by weight of sodium citrate, and 3375 parts by weight of glucose. The resulting composition can be denoted as R1, and the composition of the invention obtained hereinafter can be denoted similarly.
[0161] Preparation:
[0162] (1) Pulverizing the glucose into more than 80% of the glucose particle diameter is between 60 mesh and 80 mesh;
[0163] (2) Potassium chloride, sodium chloride, and sodium citrate are respectively pulverized into more than 80% of particle diameters between 80 mesh and 120 mesh;
[0164] (3) Get the potassium chloride powder of the recipe quantity that step (2) obtains, mix with the glucose powder that 1.5 times of weight step (1) pulverizes to obtain, and the degree of mixing reaches the [appearance uniformity] under the appendix powder of the second part of the Chinese Pharmacopoeia of the 2012 edition degree] inspection law require...
Embodiment 2
[0180] prescription: 1 part by weight of potassium chloride, 2.33 parts by weight of sodium chloride, 1.93 parts by weight of sodium citrate, and 13.3 parts by weight of glucose. The resulting composition of the invention may be designated R2.
[0181] Preparation: Carry out with reference to the method for embodiment 1, result:
[0182] (i) Agglomeration phenomenon: no agglomeration occurs in each mixing step;
[0183] (ii) mixing time: step (5) mixing required time 0.7h;
[0184] (iii) Dissolving time: the dissolving time is 85s.
Embodiment 3
[0186] prescription: 1 part by weight of potassium chloride, 2.4 parts by weight of sodium chloride, 1.6 parts by weight of sodium citrate, and 14 parts by weight of glucose.
[0187] Preparation: Carry out with reference to the method for embodiment 1, result:
[0188] (i) Agglomeration phenomenon: no agglomeration occurs in each mixing step;
[0189] (ii) mixing time: step (5) mixing required time 0.9h;
[0190] (iii) Dissolving time: the dissolving time is 90s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com