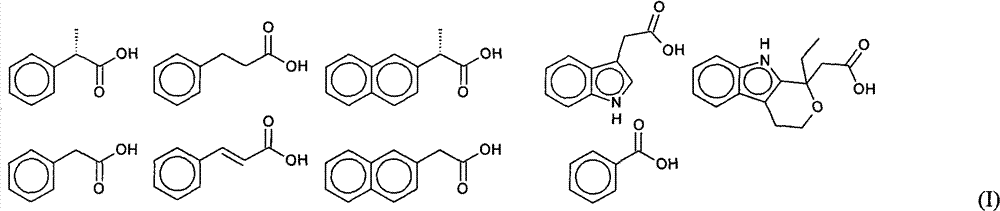
Green synthesis process of aryl methyl low-carbonate
A green synthesis technology of methyl carbonate, applied in the formation/introduction of carboxylate groups, preparation of carboxylate esters, preparation of organic compounds, etc., can solve problems such as equipment corrosion and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
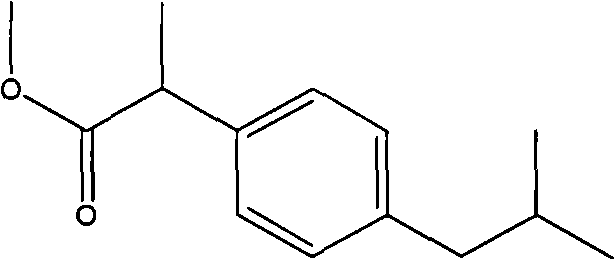
[0018] Embodiment 1, the preparation of ibuprofen methyl ester
[0019]
[0020] Take a 100mL three-neck round bottom flask, add 20.6g (0.1mol) ibuprofen, 0.2g p-toluenesulfonic acid, 40.35mL (1mol) methanol, 20mL (0.274mol) glycerin, stir, and heat the heating mantle to reflux at 70°C for 4hr. After the reaction was finished, unreacted methanol was removed by distillation under normal pressure at 75°C. Put it in a separatory funnel and let it stand for stratification, the upper layer is the methyl ester layer, and the lower layer is the glycerol layer. The glycerol layer was returned to the reaction bottle for recycling in the next round of reaction. The methyl ester layer is separated in a simulated moving bed chromatographic separation system composed of silica gel as an adsorbent and dichloromethane as an eluent to obtain ibuprofen, p-toluenesulfonic acid components and ibuprofen methyl ester components. Dichloromethane was recovered by atmospheric distillation to obt...
Embodiment 2
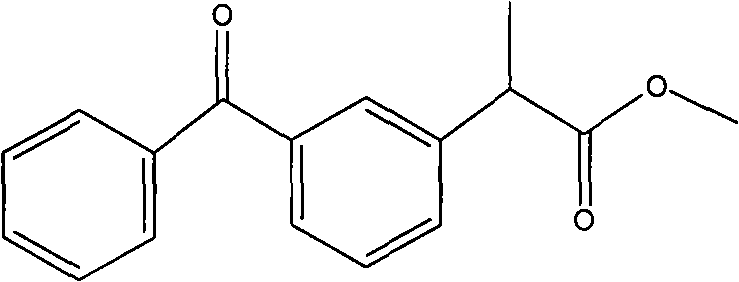
[0023] Embodiment 2, the preparation of ketoprofen methyl ester
[0024]
[0025] Take a 100mL three-neck round bottom flask, add 25.4g (0.1mol) ketoprofen, 0.5g methanesulfonic acid, 100mL (2.48mol) methanol, 50mL (0.69mol) glycerin, stir, and heat the heating mantle to reflux at 75°C for 6hr. The unreacted methanol was distilled off under reduced pressure at 30°C. Put it in a separatory funnel and let it stand for stratification, the upper layer is the methyl ester layer, and the lower layer is the glycerol layer. The glycerol layer was returned to the reaction bottle for recycling in the next round of reaction. The methyl ester layer is separated in a simulated moving bed chromatographic separation system composed of polyamide as an adsorbent and methyl tert-butyl ether as an eluent to obtain ketoprofen, methanesulfonic acid components and ketoprofen methyl ester group point. Atmospheric pressure distillation reclaimed methyl tert-butyl ether to obtain 26.01 g of keto...
Embodiment 3
[0028] The preparation of embodiment 3,2-methyl phenylpropionate
[0029]
[0030] Take a 100mL three-necked round bottom flask, add 30.40g (0.2mol) 2-phenylpropionic acid, 0.03g Novozym435, 30mL (0.74mol) methanol, 10mL (0.14mol) glycerin, stir, and heat at 55°C for 8hr. The unreacted methanol was distilled off under reduced pressure at 30°C. Filter to remove lipase, place in a separatory funnel to stand for stratification, the upper layer is the methyl ester layer, and the lower layer is the glycerol layer. The glycerol layer was returned to the reaction bottle for recycling in the next round of reaction. The methyl ester layer is separated in a simulated moving bed chromatographic separation system composed of silica gel as an adsorbent and dichloromethane as an eluent to obtain 2-phenylpropionic acid components and 2-phenylpropionic acid methyl ester components. Atmospheric pressure distillation recovered chloroform to obtain 29.90 g of methyl 2-phenylpropionate, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com