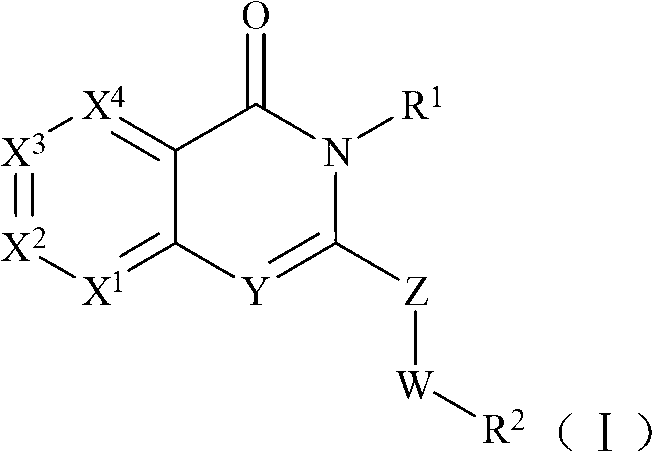
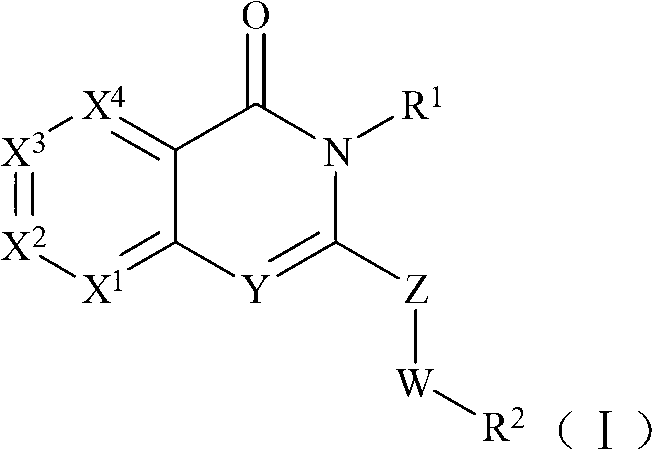
Selective phosphatidylinositol-3 kinase delta inhibitor
A compound, alkyl technology, used in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
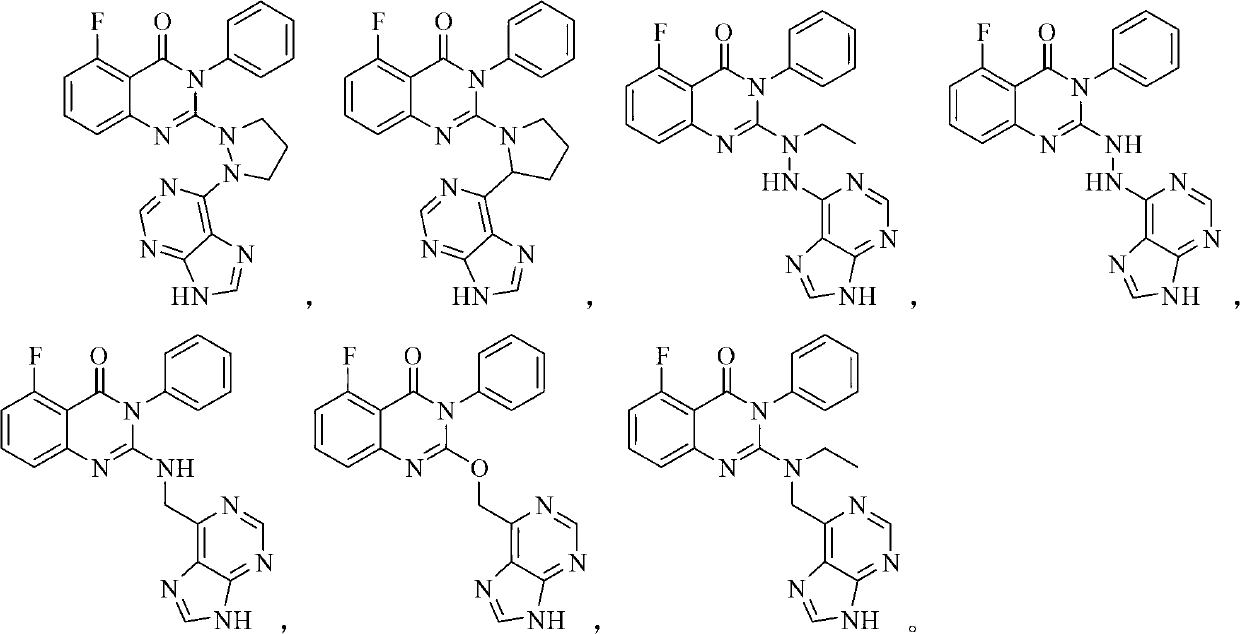
[0136] Example 1 Preparation of 2-(2-(9H-purin-6-yl)hydrazino)-5-fluoro-3-phenylquinazolin-4(3H)-one (compound 4)
[0137]
[0138] (1) Preparation of 2-fluoro-6-nitro-N-phenylbenzamide
[0139]
[0140] Dissolve 2-fluoro-6-nitrobenzoic acid (3.7 g, 20.0 mmol) in 40 mL CH 2 Cl 2 and 1.0mL DMF, slowly dropwise added oxalyl chloride (3.81g, 30.0mmol), stirred at room temperature for two hours, concentrated to remove the solvent, then dissolved in 4mL dioxane, and dropped it into aniline (1.86g ,20.0mmol), NaHCO 3 (3.36g, 40.0mmol) in dioxane (10mL) and water (10mL) solution, after the dropwise addition was completed, it was raised to room temperature and stirred for half an hour, then 200mL of water was added, a solid was precipitated, filtered by suction, and the solid was dried to obtain 5.13g of the product. Yield: 98.6%.
[0141] (2) Preparation of 2-amino-6-fluoro-N-phenylbenzamide
[0142]
[0143] Dissolve 2-fluoro-6-nitro-N-phenylbenzamide (5.2g, 20.0mmol) ...
Embodiment 2
[0164] Example 2 Preparation of 2-((9H-purin-6-yl)methylamino)-5-fluoro-3-phenylquinazolin-4(3H)-one (compound 5)
[0165]
[0166] (1) Preparation of 2-fluoro-6-nitro-N-phenylbenzamide
[0167]
[0168] Dissolve 2-fluoro-6-nitrobenzoic acid (3.7 g, 20.0 mmol) in 40 mL CH 2 Cl 2 and 1.0mL DMF, slowly dropwise added oxalyl chloride (3.81g, 30.0mmol), stirred at room temperature for two hours, concentrated to remove the solvent, then dissolved in 4mL dioxane, and dropped it into aniline (1.86g ,20.0mmol), NaHCO 3 (3.36g, 40.0mmol) in dioxane (10mL) and water (10mL) solution, after the dropwise addition was completed, it was raised to room temperature and stirred for half an hour, then 200mL of water was added, a solid was precipitated, filtered by suction, and the solid was dried to obtain 5.13g of the product. Yield: 98.6%.
[0169] (2) Preparation of 2-amino-6-fluoro-N-phenylbenzamide
[0170]
[0171] Dissolve 2-fluoro-6-nitro-N-phenylbenzamide (5.2g, 20.0mmol...
Embodiment 3
[0204] Example 3 Preparation of 2-((9H-purin-6-yl)methoxy)-5-fluoro-3-phenylquinazolin-4(3H)-one (compound 6)
[0205]
[0206] (1) Preparation of 6-chloro-9-((2-(trimethylsilyl)ethoxy)methyl)-9H-purine
[0207]
[0208] Under nitrogen protection, throw 6-chloro-9H-purine (20g, 129.40mmol) and N,N-dimethylformamide (200mL) into a 250mL four-neck round bottom flask, and hydrogenate in batches at -10°C Sodium (5.2g, 130.00mmol, content 60%), stirred for 30min. -10°C, (2-(chloromethoxy)ethyl)trimethylsilane (20.1g, 120.56mmol) was added dropwise while stirring within 20min, the resulting solution was stirred at 25°C overnight, and 1000mL of ice was added Quenched with water, extracted three times with 500 mL of ethyl acetate. The combined organic layers were washed three times with 50 mL of brine, dried over anhydrous sodium sulfate, concentrated in vacuo, and subjected to silica gel column chromatography (ethyl acetate:petroleum ether=1:10) to obtain a yellow oil (22 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com