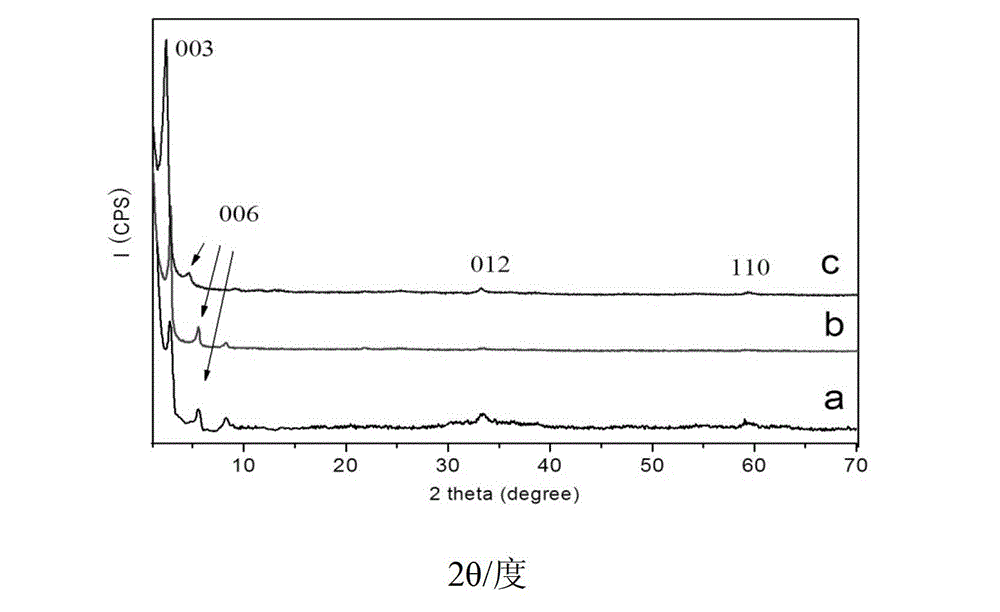
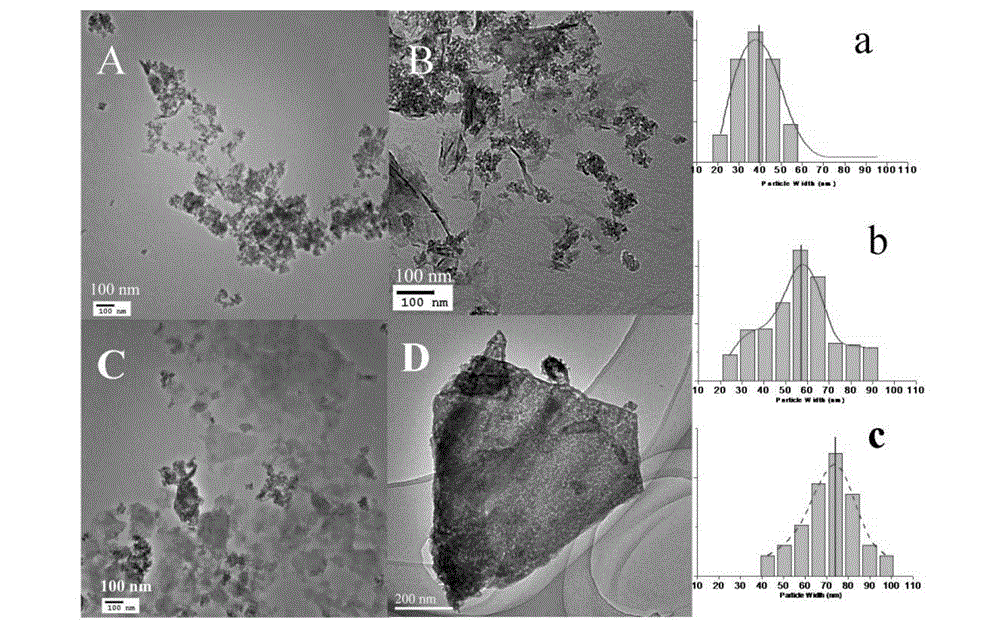
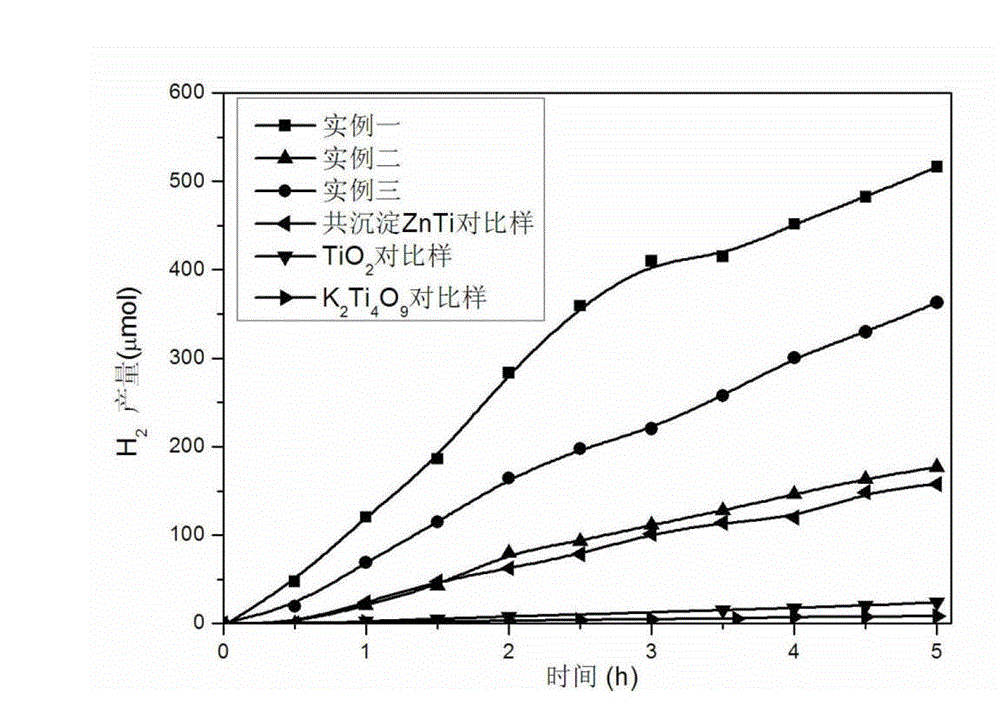
ZnTi hydrotalcite nanosheet catalyst and application of catalyst in hydrogen preparation by photoactivating and decomposing water
A nanosheet and catalyst technology, which is applied in the field of ZnTi hydrotalcite nanosheet catalyst and its photocatalytic water splitting to produce hydrogen, can solve the problems of low photocatalytic efficiency, achieve excellent semiconductor characteristics, simple synthesis conditions, and easy large-scale industrial production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1. Configure microemulsion: Based on a 100ml three-necked flask, add 50ml of isopropanol, 1.1ml of deionized water, and 1.08g of surfactant sodium lauryl sulfate into the three-necked flask, stir; slowly add 1-butanol 2ml, stir until the solution is clear;
[0018] 2. Add 0.002mol of Zn(NO 3 ) 2 ·6H 2 O was added to the microemulsion prepared in step 1, and after the salt was dissolved, TiCl was added in a closed environment 4 Solution 0.11ml, after the smoke disappears, add urea 0.900g, crystallize and reflux at 90°C for 24h; the added TiCl 4 The solution contains 0.001mol TiCl 4 ;
[0019] 3. After the reaction is completed, the product is suction-filtered, centrifuged and washed 3 times with a mixed solution of deionized water and ethanol with a volume ratio of 1:1, and then washed 3 times with absolute ethanol, and the filter cake is dried in an oven at 60°C for 12 hours to obtain ZnTi hydrotalcite nanosheet catalyst.
[0020] The chemical formula of the ZnTi...
Embodiment 2
[0024] 1. Configure microemulsion: Based on a 100ml three-necked flask, add 50ml of isopropanol, 1.1ml of deionized water, and 0.72g of surfactant sodium lauryl sulfate into the three-necked flask, stir; slowly add 1-butanol 2ml, stir until the solution is clear;
[0025] 2. Add 0.002mol of Zn(NO 3 ) 2 ·6H 2 O was added to the microemulsion prepared in step 1, and after the salt was dissolved, TiCl was added in a closed environment 4 Solution 0.11ml, after the smoke disappears, finally add urea 0.900g, crystallize and reflux at 90°C for 24h; the added TiCl 4 The solution contains 0.001mol TiCl 4 ;
[0026] 3. After the reaction is completed, the product is suction-filtered, centrifuged and washed 3 times with a mixed solution of deionized water and ethanol with a volume ratio of 1:1, and then washed 3 times with absolute ethanol, and the filter cake is dried in an oven at 60°C for 12 hours to obtain ZnTi hydrotalcite nanosheet catalyst.
[0027] The chemical formula of ...
Embodiment 3
[0031] 1. Configure microemulsion: Based on a 100ml three-necked flask, add 50ml of isopropanol, 1.1ml of deionized water, and 0.36g of surfactant sodium lauryl sulfate into the three-necked flask, stir; slowly add 1-butanol 1.5ml, stir until the solution is clear;
[0032] 2. Add 0.002mol of Zn(NO 3 ) 2 ·6H 2 O was added to the microemulsion prepared in step 1, and after the salt was dissolved, TiCl was added in a closed environment 4 Solution TiCl 4 0.11ml, after the smoke disappears, add urea 0.900g at last, crystallize and reflux at 90°C for 24h; the added TiCl 4 The solution contains 0.001mol TiCl 4 ;
[0033] 3. After the reaction is completed, the product is suction-filtered, centrifuged and washed 3 times with a mixed solution of deionized water and ethanol with a volume ratio of 1:1, and then washed 3 times with absolute ethanol, and the filter cake is dried in an oven at 60°C for 12 hours to obtain ZnTi hydrotalcite nanosheet catalyst.
[0034] The chemical f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com