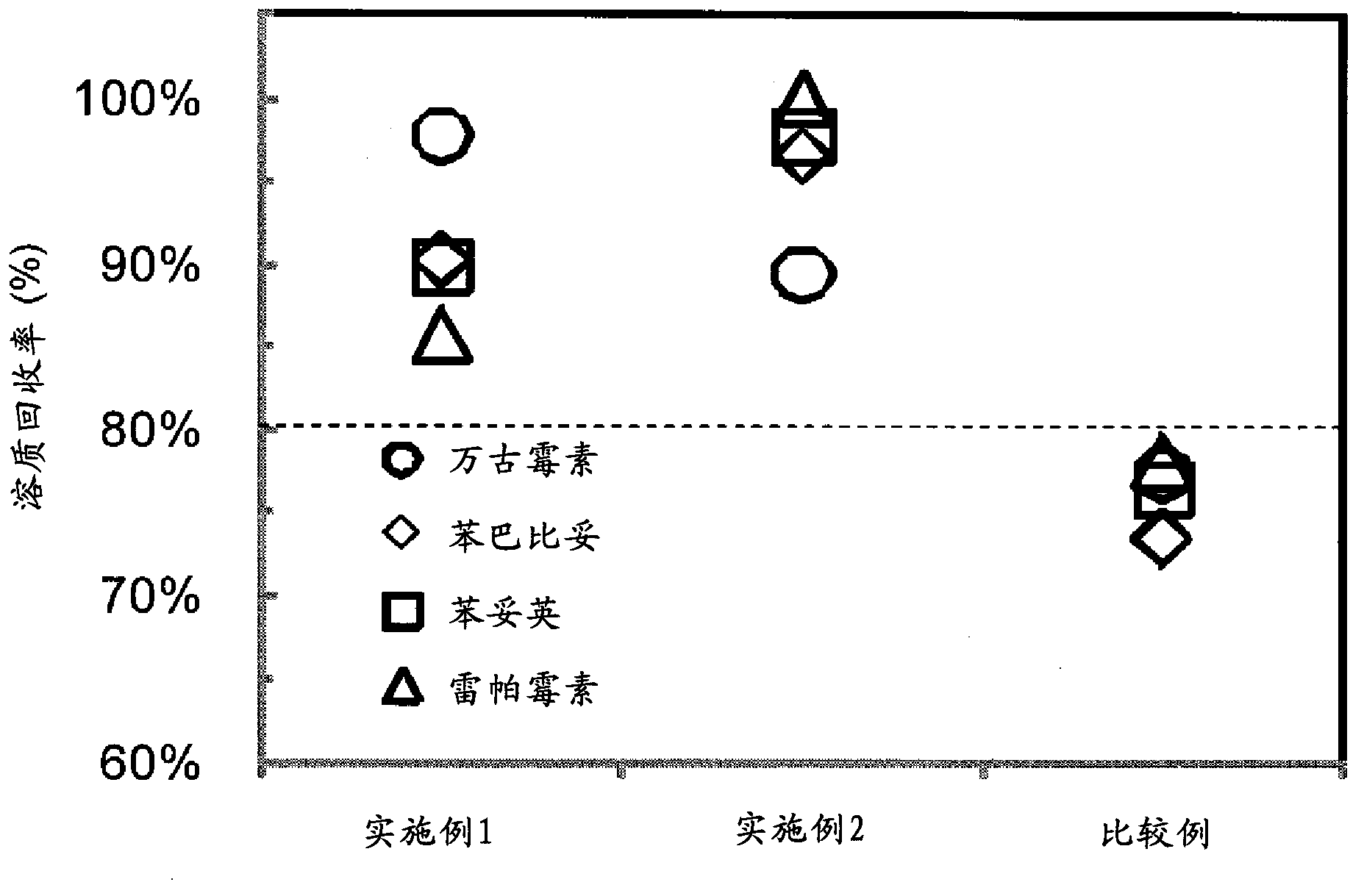
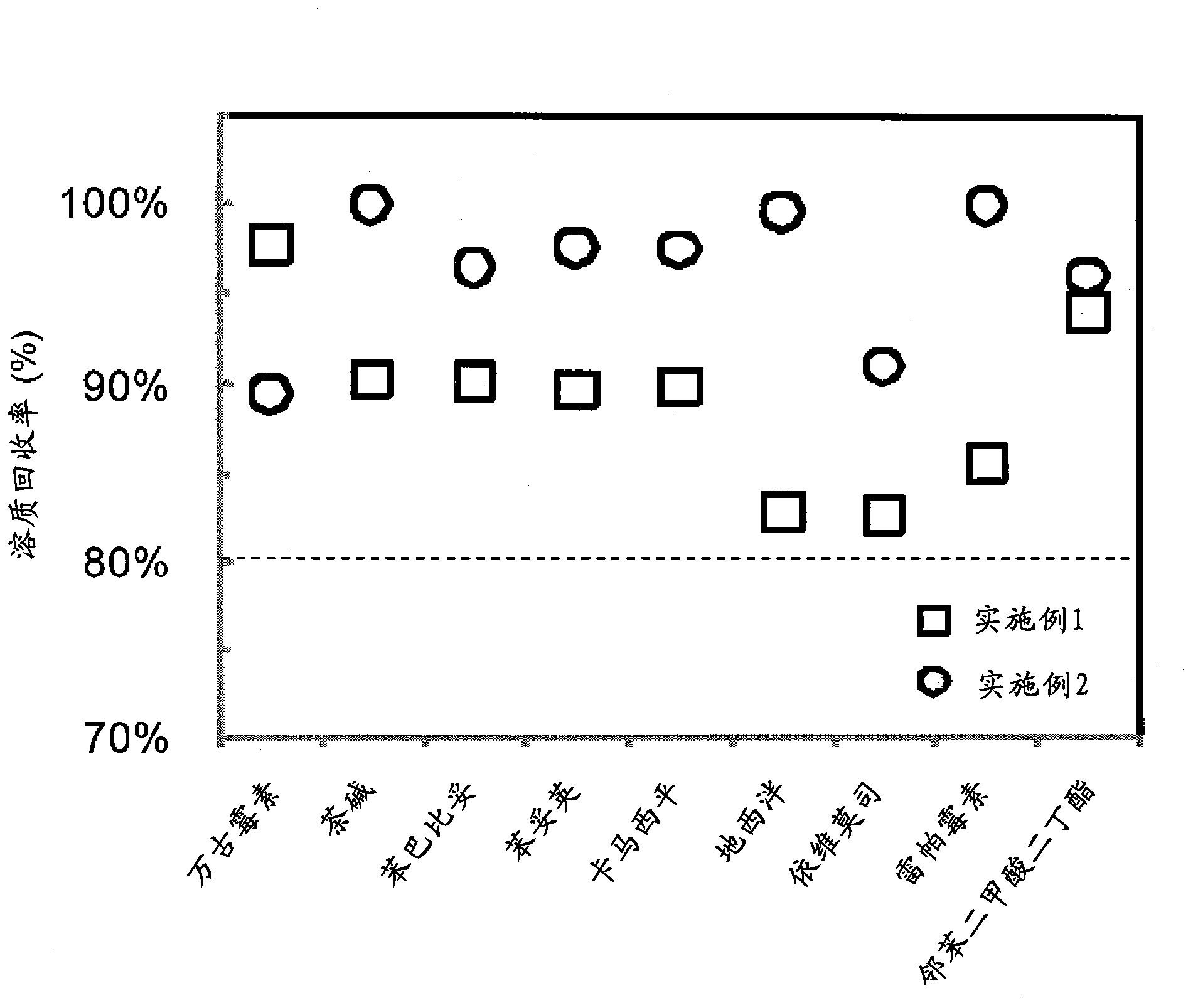
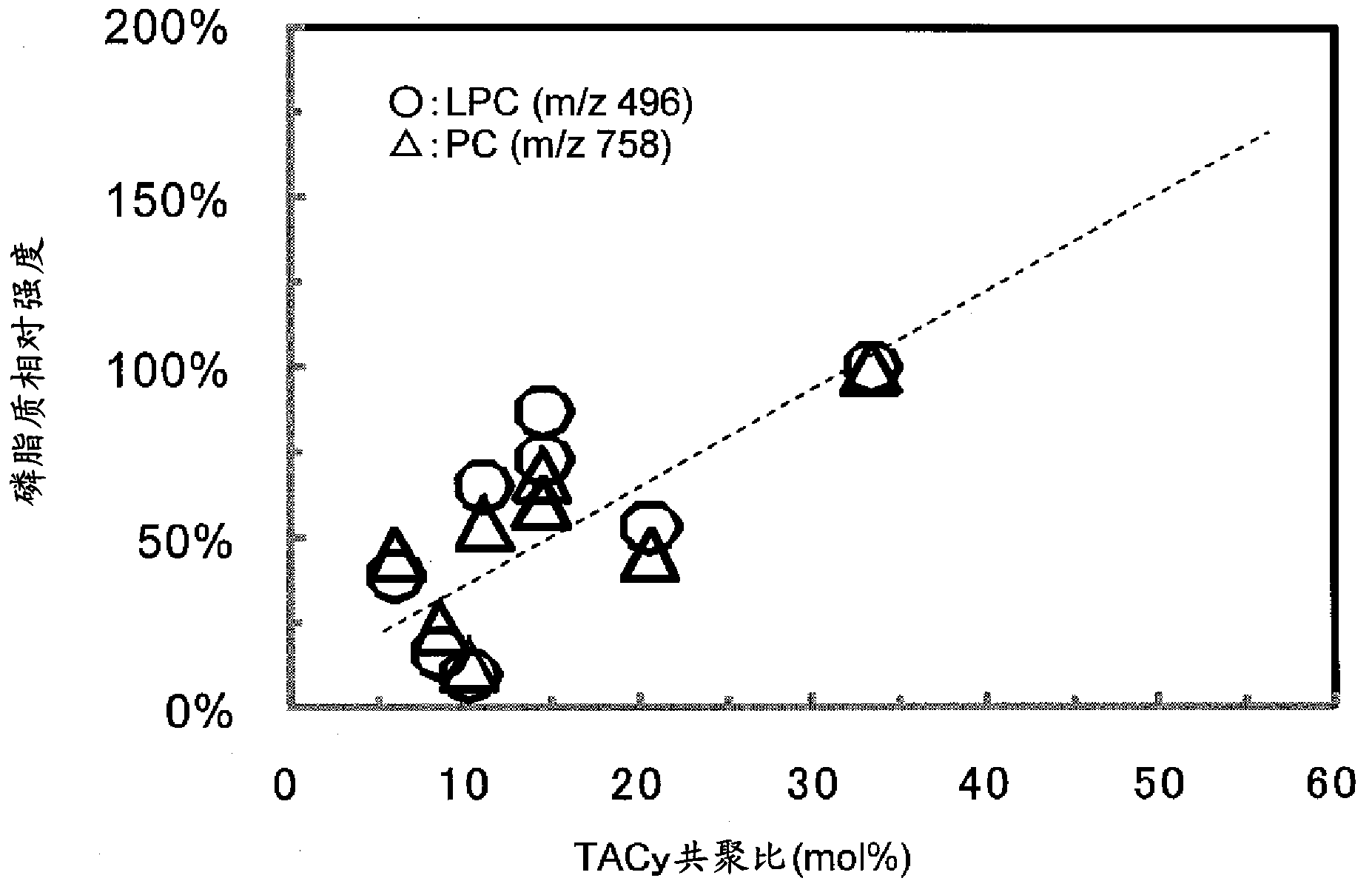
Adsorbent and method for producing same
A technology of adsorption materials and monomers, which is applied in the direction of material separation, analytical materials, chemical instruments and methods, etc., and can solve the problems of decreased retention capacity and inability to show solid phase extraction performance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0272] (Example 1) Preparation of Divinylbenzene-Triallyl Isocyanurate Copolymer
[0273] A 500 mL separable flask was charged with 2.0 g of hydroxypropyl cellulose (HPC, manufactured by Aldrich, average molecular weight ~ 10,000, viscosity 5 cP (2 wt% aqueous solution, 20°C)) and 100 mL of water, and stirred until completely dissolved. Next, 7.84 g (0.06 mol) of divinylbenzene (DVB, manufactured by Aldrich, a mixture of 80% divinylbenzene + 19% ethylvinylbenzene), triallyl isocyanurate (TAIC, Tokyo Chemical Industry Co., Ltd.) were mixed. 14.95 g (0.06 mol) of toluene (manufactured by Wako Pure Chemical Industries, Ltd.), 11.5 g of toluene (manufactured by Wako Pure Chemical Industries, Ltd.), and 0.22 g of azobisisobutyronitrile (AIBN, manufactured by Tokyo Chemical Industry Co., Ltd.) were completely dissolved and put into a separable flask. The nitrogen introduction pipe and the cooling pipe were connected to the separable flask, and the polymerization system was stirred f...
Embodiment 2)
[0274] (Example 2) Preparation of Divinylbenzene-Triallyl Cyanurate Copolymer (1)
[0275] A 500 mL separable flask was charged with 2.0 g of hydroxypropyl cellulose (HPC, manufactured by Aldrich, average molecular weight ~ 10,000, viscosity 5 cP (2 wt% aqueous solution, 20°C)) and 100 mL of water, and stirred until completely dissolved. Next, 7.8 g (0.06 mol) of divinylbenzene (DVB, manufactured by Aldrich, a mixture of 80% divinylbenzene + 19% ethylvinylbenzene), triallyl cyanurate (TACy, manufactured by Tokyo Chemical Industry Co., Ltd.) were mixed. ) 15.0 g (0.06 mol), 11.5 g of toluene (manufactured by Wako Pure Chemical Industries, Ltd.), and 0.2 g of azobisisobutyronitrile (AIBN, manufactured by Tokyo Chemical Industry Co., Ltd.), were completely dissolved, and then placed in a separable flask. The nitrogen introduction pipe and the cooling pipe were connected to the separable flask, and the polymerization system was stirred for 30 minutes using a stirring blade while p...
Embodiment 3)
[0276] (Example 3) Preparation of Divinylbenzene-Triallyl Cyanurate Copolymer (2)
[0277] A 500 mL separable flask was charged with 6.0 g of hydroxypropyl cellulose (HPC, manufactured by Aldrich, average molecular weight ~ 10,000, viscosity 5 cP (2 wt% aqueous solution, 20°C)) and 200 mL of water, and stirred until completely dissolved. Next, 12.5 g (0.10 mol) of divinylbenzene (DVB, manufactured by Aldrich, a mixture of 80% divinylbenzene + 19% ethylvinylbenzene), triallyl cyanurate (TACy, manufactured by Tokyo Chemical Industry Co., Ltd.) were mixed. ) 6.0 g (0.02 mol), 8.0 g of toluene (manufactured by Wako Pure Chemical Industries, Ltd.), and 0.2 g of azobisisobutyronitrile (AIBN, manufactured by Tokyo Chemical Industry Co., Ltd.), were completely dissolved, and then placed in a separable flask. The nitrogen introduction pipe and the cooling pipe were connected to the separable flask, and the polymerization system was stirred for 30 minutes using a stirring blade while pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com