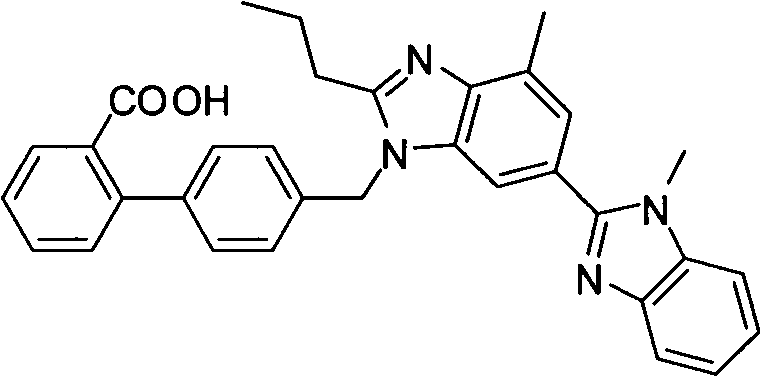
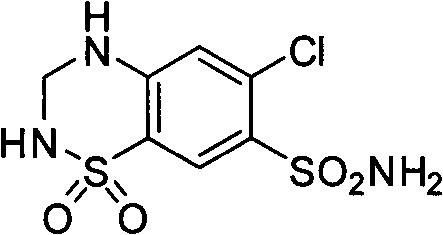
Preparation method of compound preparation for treating high blood pressure
A compound and co-solvent technology, used in pill delivery, cardiovascular system diseases, drug combinations, etc., can solve problems such as reducing bioavailability and reducing drug dissolution rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029]
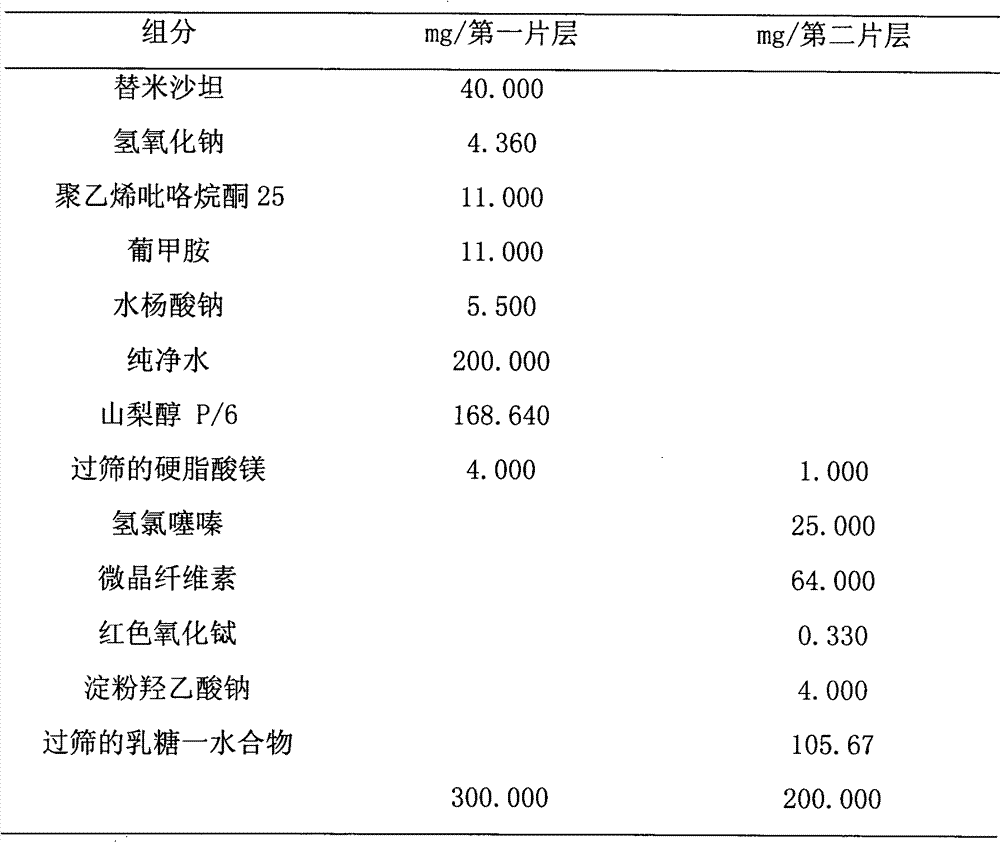
[0030] Preparation:
[0031] 1. Preparation of the final mixture of the first sheet.
[0032] At 20°C, add 200.0kg of purified water, 3.0kg of sodium hydroxide, 6.0kg of sodium salicylate, 45.0kg of telmisartan, 14.0kg of povidone K, and 13.5kg of meglumine into a stainless steel container. Dubbed into a light yellow solution, set aside.
[0033] The above solution is sprayed into a suitable spray dryer and spray dried to obtain white to off-white fine particles, which are sieved to obtain the final mixture of the above first sheet.
[0034] 2. The final mixture of the second sheet.
[0035] Mix 9.0kg of purified water at 70°C, 6.0kg of starch, 112.0kg of lactose monohydrate, 12.5kg of hydrochlorothiazide, 64.0kg of microcrystalline cellulose sodium, and 0.330kg of red iron oxide until homogeneous to obtain the final product of the second sheet. mixture.
[0036] 3. Preparation of bilayer tablet.
[0037] Using a suitable tablet press, 240 kg of the final blen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com