Luminescent solar concentrator comprising disubstituted benzothiadiazole compounds
A technology for emitting solar energy and benzothiadiazole, which can be applied in electroluminescence light sources, luminescent materials, chemical instruments and methods, etc., and can solve problems such as small absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
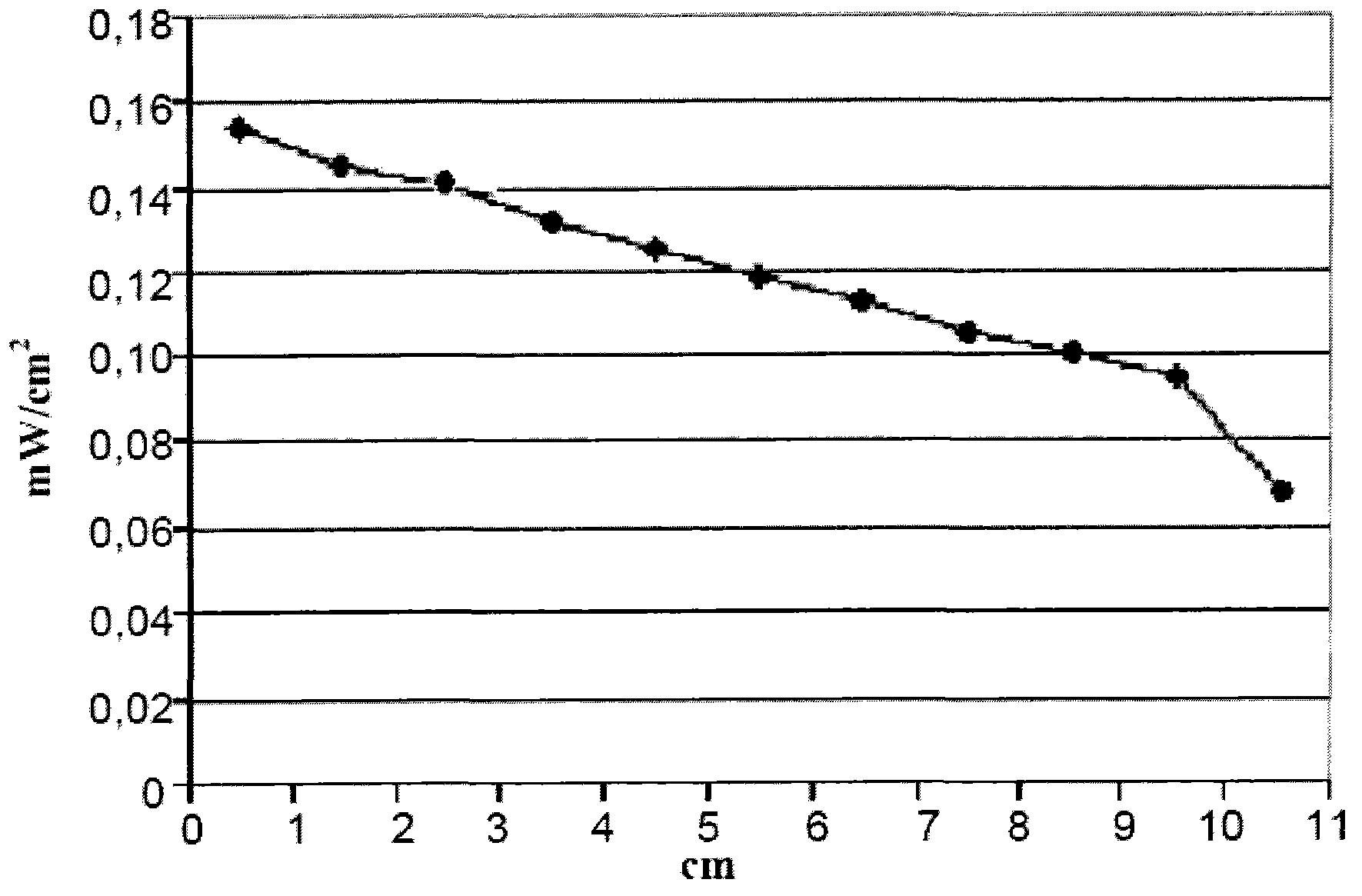
[0064] 6g of polymethylmethacrylate Altuglas VSUVT 100 (PMMA) and 76.5mg of 4,7-bis(2',2"-dithiophen-5'-yl)-2,1,3-benzothiadiazole (QTB ) was dissolved in 30ml of 1,2-dichlorobenzene. Subsequently, the resulting solution was uniformly deposited on a polymethyl methacrylate plate Almglas VSUVT 100 (PMMA) (size 90x90x6mm) using a doctor blade type film coater, and The solvent was allowed to evaporate for 24 hours at room temperature (25° C.) in a small air stream. A red transparent plate (plate 1 ) was obtained, the color being imparted by a thin film whose thickness proved to range from 50 μm to 350 μm.
[0065] Then, will have 1.2cm 2 A surface photovoltaic cell IXYS-XOD17 is applied to one of the edges of the polymer plate.
[0066] Then, with 1sun (1000W / m 2 ) light source of power to illuminate the main side of the polymer plate [containing 4,7-bis(2,,2"-dithiophen-5'-yl)-2,1,3-benzothiadiazole (QTB ) of the film-covered side] and measure the electrical power generated b...
example 2
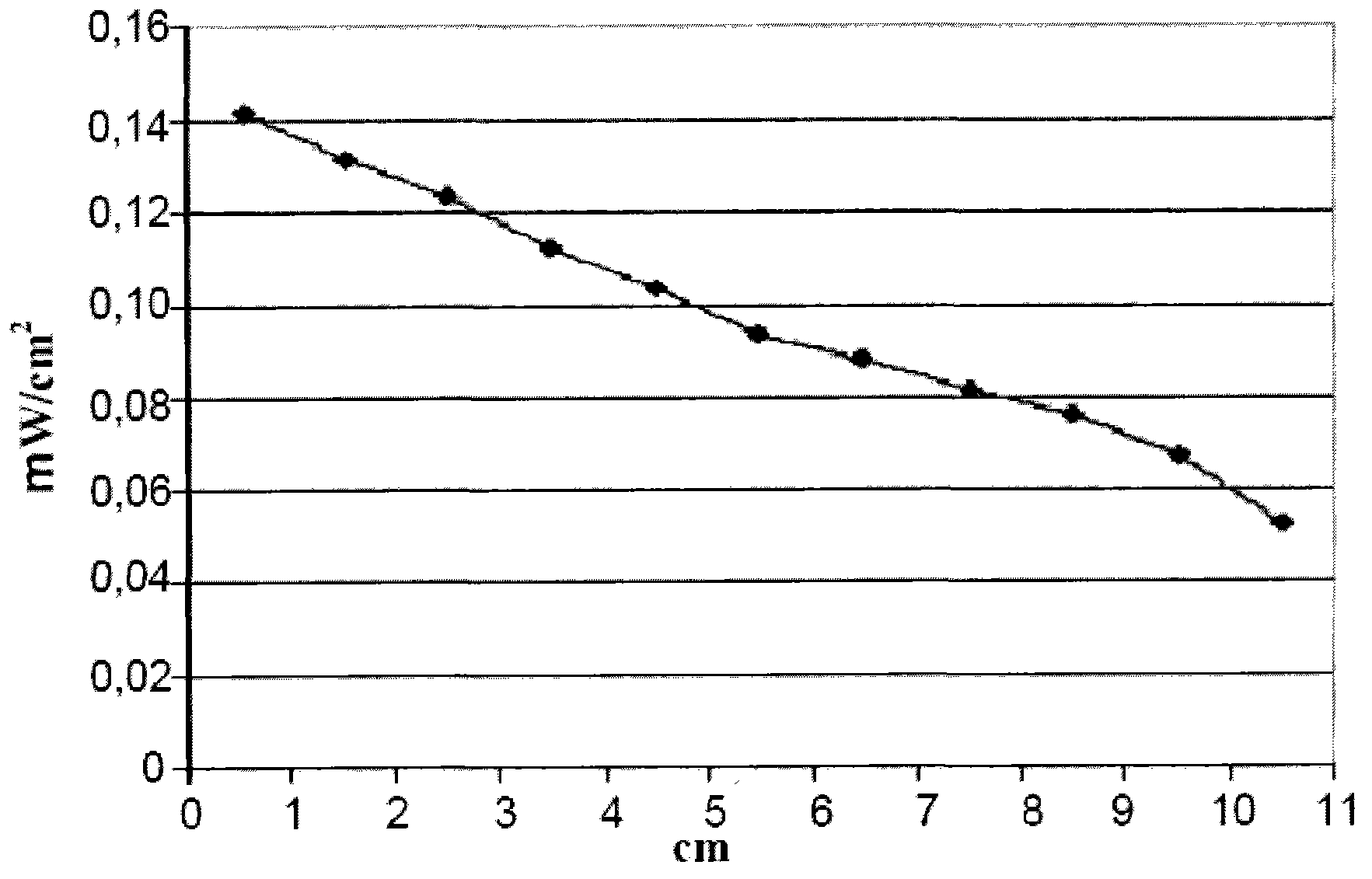
[0071] 6g of polymethylmethacrylate Almglas VSUVT 100 (PMMA) and 104.2mg of 4,7-bis(5"-n-hexyl-2,,2"-dithiophene-5'-yl)-2,1,3-benzene Thiadiazole (QTB-ex) was dissolved in 30ml of 1,2-dichlorobenzene. Then, the resulting solution was uniformly deposited on a plate (dimensions 90x90x6 mm) of polymethylmethacrylate Altuglas VSUUT 100 (PMMA) using a doctor blade type film coater, and the solvent was allowed to cool at room temperature (25° C.) in a small Evaporate in a stream of air for 24 hours. A red transparent plate (plate 2) was obtained, the color being imparted by a thin film, the thickness of which proved to range from 50 μm to 350 μm.
[0072] Then, will have 1.2cm 2 A surface photovoltaic cell IXYS-XOD17 is applied to one of the edges of the polymer plate.
[0073] Then, with 1sun (1000W / m 2 ) light source of power to illuminate the main side of the polymer plate [contained 4,7-bis(5"-n-hexyl-2,,2"-dithiophen-5'-yl)-2,1,3-benzene Thiadiazole (QTB-ex) film-covered s...
example 3
[0077] Example 3 (comparative)
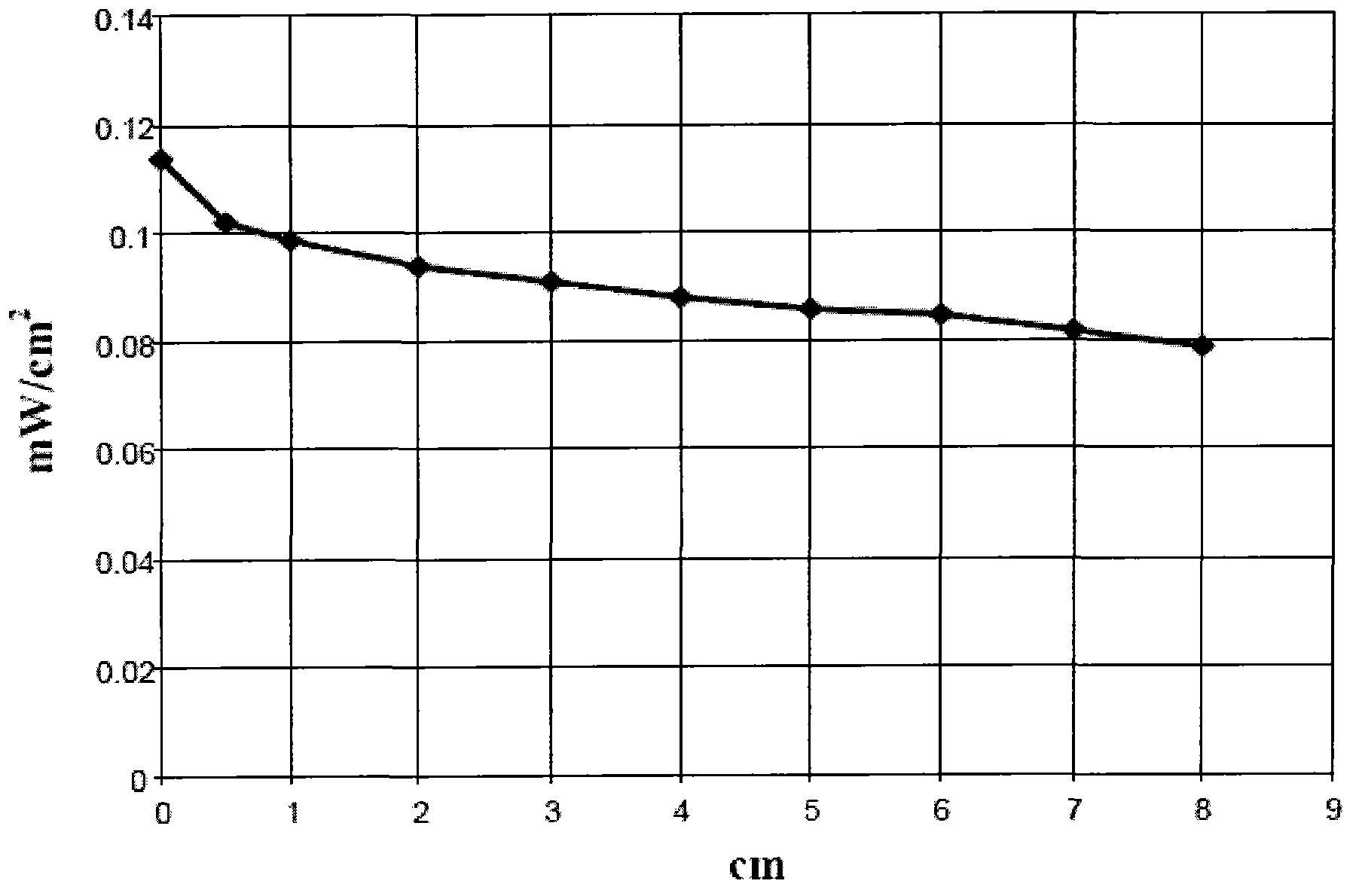
[0078] 6 g of polymethyl methacrylate Altuglas VSUVT 100 (PMMA) and 49.5 mg of 4,7-di(thiophen-2'-yl)-2,1,3-benzothiadiazole (DTB) were dissolved in 30 ml of 1, 2-dichlorobenzene. Then, the resulting solution was uniformly deposited on a plate (dimensions 90x90x6 mm) of polymethylmethacrylate Altuglas VSUUT 100 (PMMA) using a doctor blade type film coater, and the solvent was allowed to cool at room temperature (25° C.) in a small Evaporate in a stream of air for 24 hours. A yellow transparent plate (plate 3) was obtained, the color being imparted by a thin film, the thickness of which proved to range from 50 μm to 350 μm.
[0079] Then, will have 1.2cm 2 A surface photovoltaic cell is applied to one of the edges of the polymer sheet.
[0080] Then, with 1sun (1000W / m 2 ) of power illuminates the main side of the polymer plate [the side covered by the film containing 4,7-bis(thiophen-2'-yl)-2,1,3-benzothiadiazole], and measures Electric...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com