Method for producing fluorine compound
A manufacturing method and technology of aluminum fluoride, applied in organic chemical methods, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as reactions that are not suitable for flow-through
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] (Manufacture of Catalyst A)
[0063] 18 ml of alumina was added to a reaction tube with a diameter of 14 mm and a length of 300 mm. Under a nitrogen flow of 100 ml / min, the reaction tube was heated to 400°C. Next, dichlorodifluoromethane (CFC-12) was passed through at 400° C. and 100 ml / min for 3 hours to obtain catalyst A. Catalyst A has a surface area of 72.56m 2 / g.
[0064] (Manufacture of hexafluoro-2-butyne)
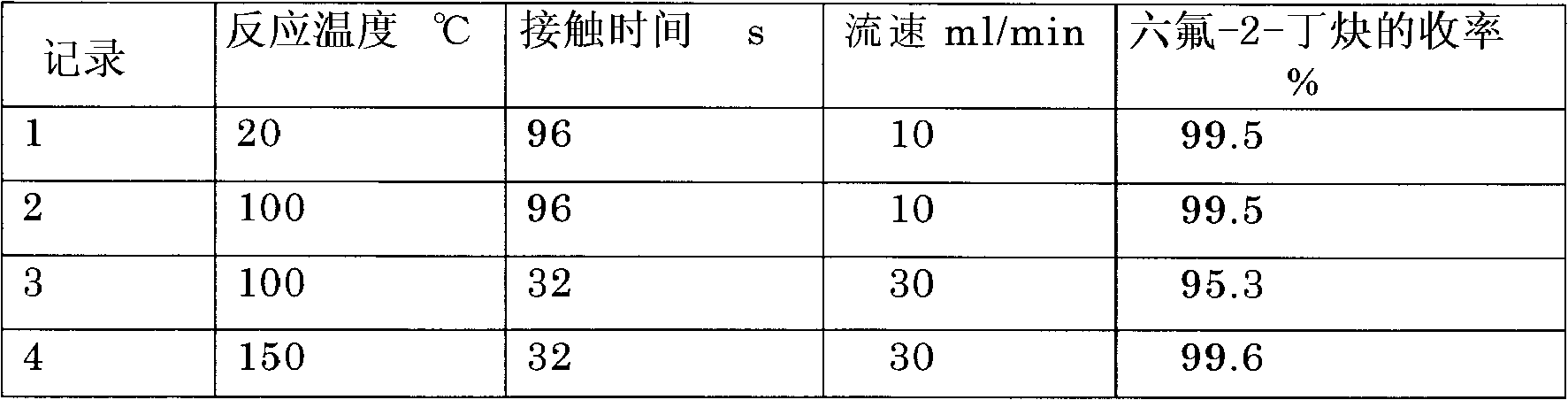
[0065] Catalyst A was added to the above reaction tube. Measure hexafluoro-1,3-butadiene with a mass flow meter and pass it into the reaction tube. The reaction is carried out at 20°C to 150°C, the product passes through a dryer and online GC, and the final product is collected in a trap at -100°C.
[0066] The experimental results are shown in Table 1 below.
[0067] [Table 1]
[0068]
Embodiment 2
[0070] (Manufacture of Catalyst B)
[0071] A sufficient amount of palladium chloride solution was impregnated into the porous aluminum fluoride (PAF) overnight. The amount of metal chloride in the solution was adjusted so that the final metal loading was about 3% by weight. After impregnation, the palladium-loaded carrier was heated at 200°C for 6 hours, further heated at 300°C for 6 hours, and then reduced at 200°C for 6 hours under a flow of hydrogen gas at a flow rate of 20ml / min, and then reduced at 300°C. After 6 hours, it was further reduced at 350°C for 5 hours to obtain catalyst B.
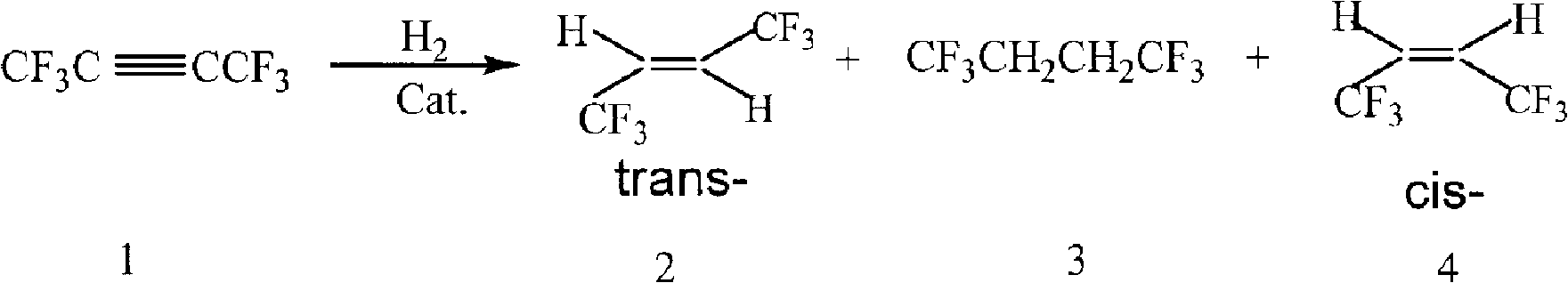
[0072] (Manufacture of cis-1,1,1,4,4,4-hexafluoro-2-butene)
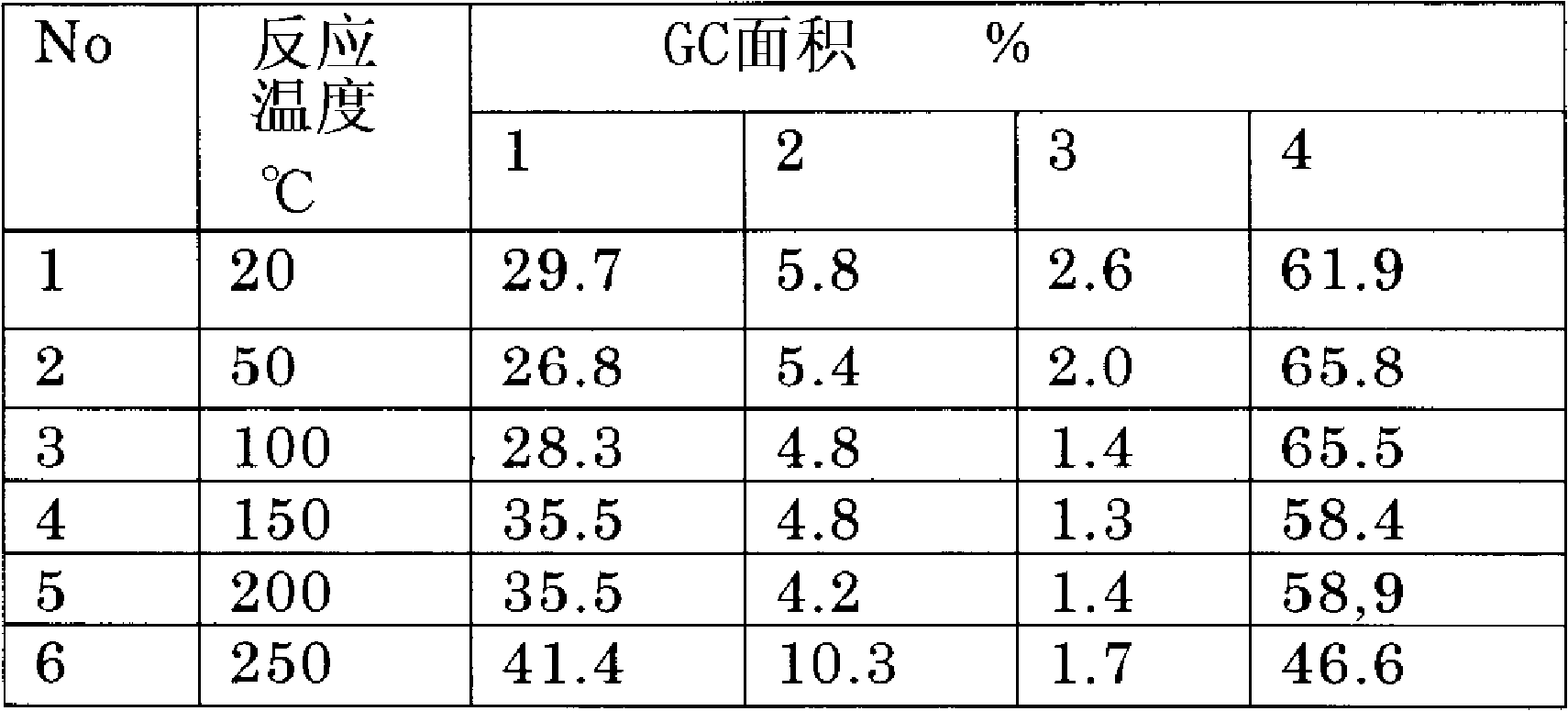
[0073] Catalyst B was added to the above reaction tube. Measure hexafluoro-2-butyne with a mass flow meter and pass it into the reaction tube. The reaction is carried out at 20°C to 250°C, the product passes through a dryer and online GC, and the final product is collected in a trap at -100°C.
[0074] The experimental res...
Embodiment 3
[0083] (Manufacture of Catalyst C)
[0084] A sufficient solution of palladium chloride and bismuth chloride was impregnated into porous aluminum fluoride (PAF) overnight. The amount of the metal chloride in the solution was adjusted so that the supporting amount of palladium became about 2% by weight and the supporting amount of bismuth became about 0.1%. The palladium-loaded carrier was heated at 200°C for 6 hours, further heated at 300°C for 6 hours, and then reduced at 200°C for 6 hours and at 300°C for 6 hours under a flow of hydrogen gas at a flow rate of 20ml / min. It was further reduced at 350° C. for 5 hours to obtain catalyst C.
[0085] (Manufacture of cis-1,1,1,4,4,4-hexafluoro-2-butene)
[0086] Catalyst C was added to the above reaction tube. Measure hexafluoro-2-butyne with a mass flow meter and pass it into the reaction tube. The reaction is carried out at 20°C to 250°C, the product passes through a dryer and online GC, and the final product is collected in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com