Heterodimer binding proteins and uses thereof
A peptide heterodimer and specific binding technology, which is applied in the field of preparation and use of the polypeptide heterodimer, can solve the problem that the immunoglobulin fusion technology does not provide a heterodimeric protein production method, cannot be actively operated, and the protein does not contain And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 9 and 10)
[0217] In certain embodiments, the hinge sequence is plasma and serum stable and resistant to proteolytic cleavage. The first lysine in the upper hinge region of IgG1 can be mutated to minimize proteolytic cleavage, e.g. lysine can be replaced with methionine, threonine, alanine or glycine or deleted (see, e.g. SEQ ID NO:379-434, which may contain a linking amino acid at the amino terminus, eg RT).
[0218] In some embodiments, the hinge sequence may contain a naturally occurring or added motif capable of forming one or more disulfide bonds to stabilize the carboxy terminus of the molecule, such as the immunoglobulin hinge core structure CPPCP (SEQ ID NO:228). In other embodiments, the hinge sequence may contain one or more glycosylation sites.
[0219] Exemplary hinges, including altered immunoglobulin hinges, are shown in SEQ ID NOs: 379-434, 618-749, and 763-791.
[0220] In certain embodiments, the single-chain polypeptides of polypeptide heterodimers according to the in...
Embodiment 1
[0371] Bivalent polypeptide heterodimers containing two pairs of heterodimerization domains
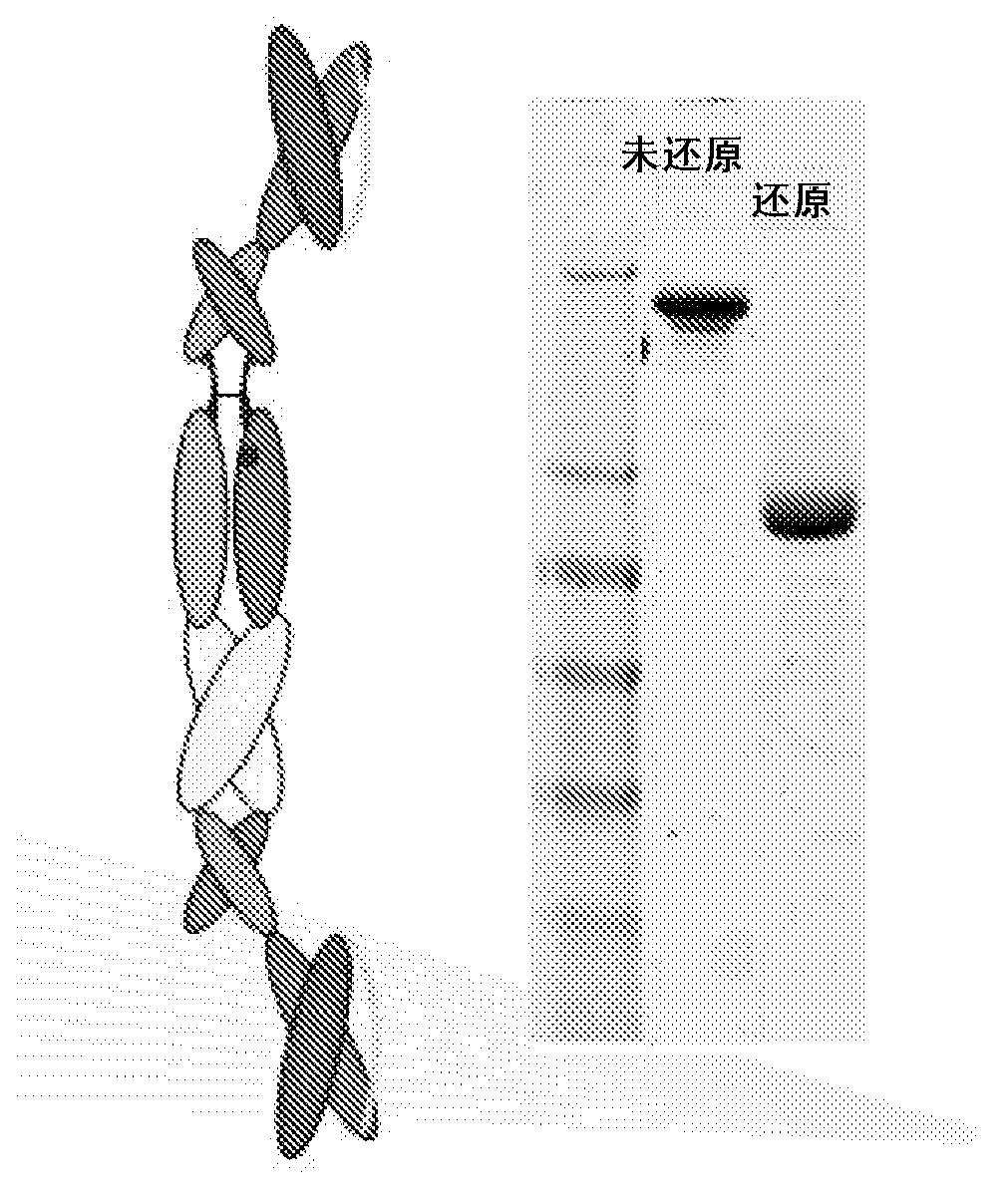
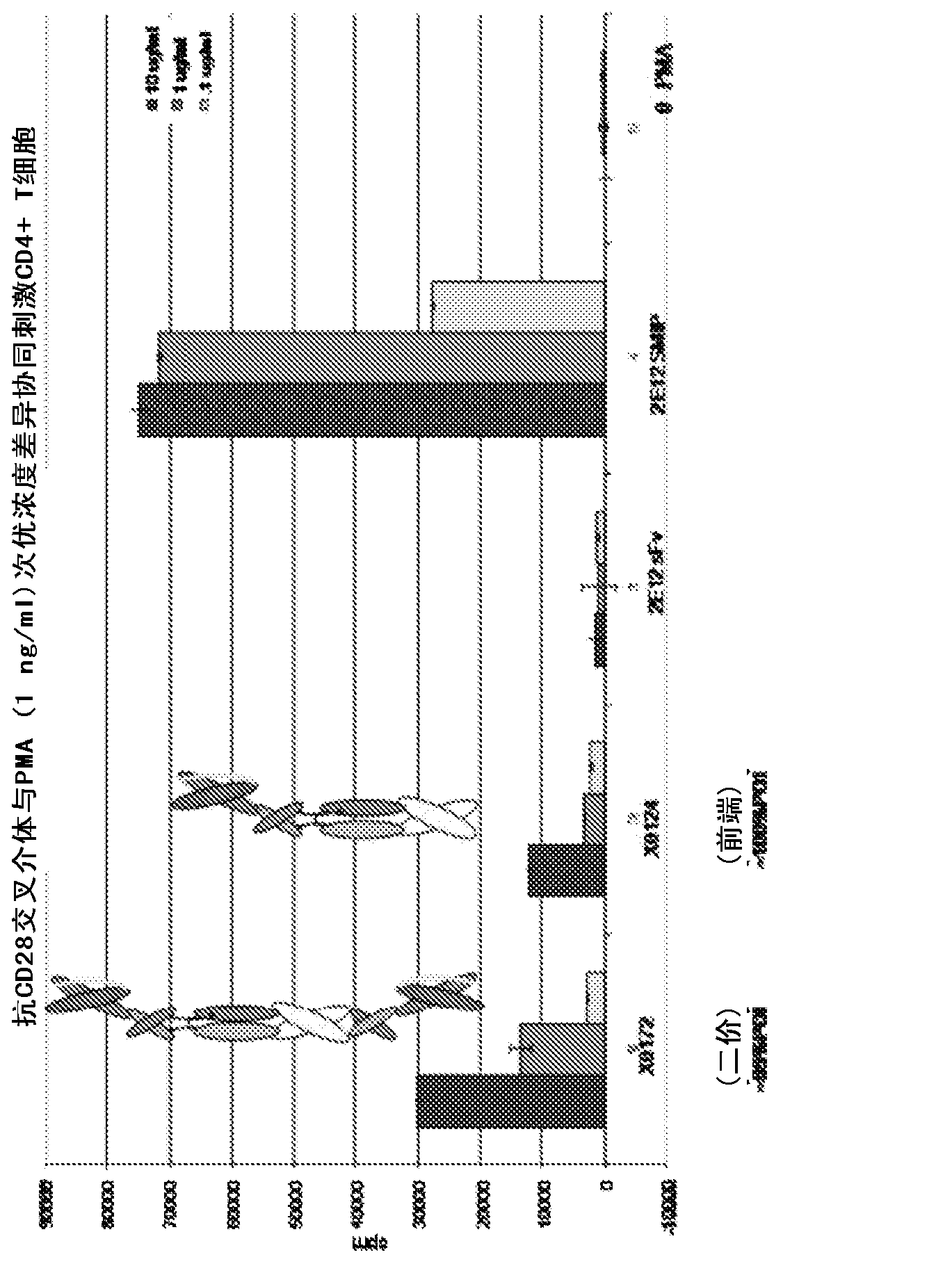
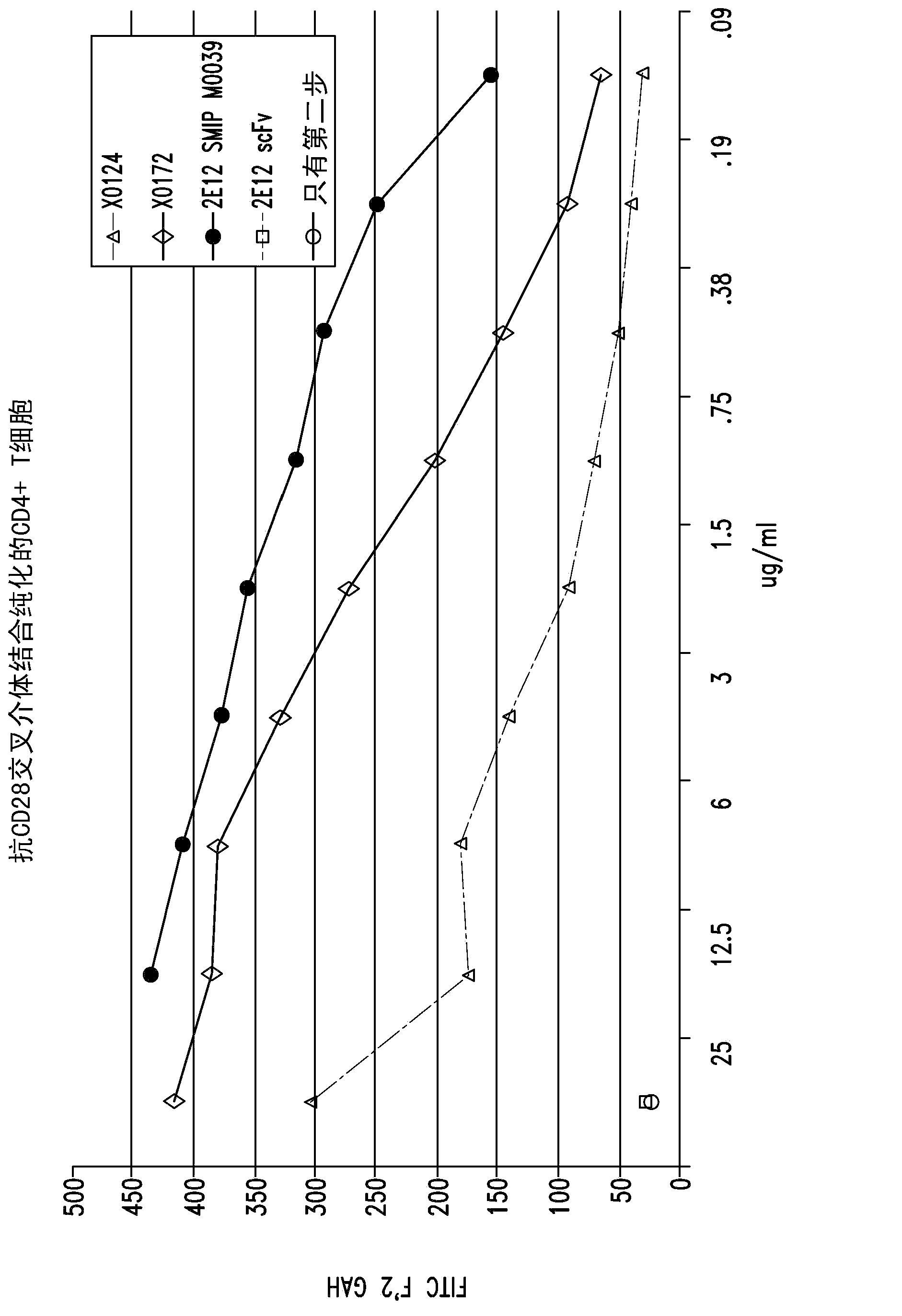
[0372] The polypeptide heterodimer X0172 was generated by co-expressing single-chain polypeptides X0130 (2E12 CH1 CH2 CH3 Ck) and X0168 (Ck CH2 CH3 CH1 H68 2E12). Single-chain polypeptide X0130 contains from its amino to carboxyl terminus: 2E12 (anti-CD28) scFv, human IgG1 CH1, human IgG1 SCC-P hinge, human IgG1 CH2, human IgG1 CH3 and altered human Ck (without the first Arg or the last Cys). The nucleotide and amino acid sequences of X130 are shown in SEQ ID NO: 1 and 2, respectively. Single-chain polypeptide X0168 contains from its amino to carboxyl terminus: altered human Ck (without the first Arg or last Cys), human IgG1 SCC-P hinge, human IgG1 CH2, human IgG1 CH3, human IgG1 CH1, H68 linker and 2E12 (anti-CD28) scFv. The nucleotide and amino acid sequences of X0168 are shown in SEQ ID NO: 3 and 4, respectively. The amino acid sequence of the H68 linker is shown in SEQ ID NO:7...
Embodiment 2
[0387] Bivalent, trivalent, and tetravalent polypeptide heterodimers using a single pair of heterodimerization domains
[0388] The bivalent polypeptide heterodimer X0251 was generated by co-expressing single-chain polypeptides X0244 (Ck(YAE)CH2(N297A)CH3 H68 2E12) and X0245(2E12 CH1 CH2(N297A)CH3). The single-chain polypeptide X0244 contains from its amino to carboxyl terminus: human Ck (YAE) (for example, human Ck without the first Arg or the last Cys, but with N30Y, V55A and T70E substitutions), human IgG1 SCC-P hinge, Human IgG1 CH2(N297A) (eg, human IgG1 CH2 with N297A substitution), human IgG1 CH3, H68 linker and 2E12 (anti-CD28) scFv. The nucleic acid and amino acid sequences of X0244 are shown in SEQ ID NO: 9 and 10, respectively. The single-chain polypeptide X0245 contains from its amino to carboxyl terminus: 2E12 (anti-CD28) scFv, human IgG1 CH1, human IgG1 SCC-P hinge, human IgG1 CH2 (N297A) and human IgG1 CH3. The nucleic acid and amino acid sequences of X0245 ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com