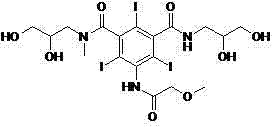
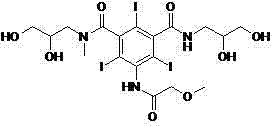
Novel preparation method of iopromide
A technology for iopromide and triiodoisophthalic acid is applied in the field of preparation of obtaining two kinds of iopromide, can solve the problems such as the inability to improve the yield of the synthesis process, and achieves the effects of solving color and luster and removing bismer by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
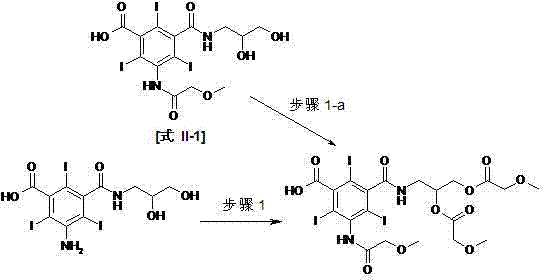
[0061] 5-amino-3-(2,3-dihydroxypropylacetyl)-2,4,6-triiodoisophthalic acid (63.2 g, 100mol), methoxyacetic anhydride (97.2 g, 600 mmol ), DMAP (0.63 g, 5.1 mmol) were mixed and heated to 100 ° C, stirred for 20 hours, and the reaction of the raw materials was basically completed. After washing with solvent, 74.1 g of the title compound was obtained as a white solid, with an HPLC purity ≥ 98% and a yield of 85.6%.
[0062] LC-MS: 848.8 ( theoretical value 848.13). 1 H NMR (DMSO-d 6 , 500MHz) 13.98(s, 1H); 10.11~10.00(2s, 1H); 8.95~8.82(m, 1H); 5.22~5.18(m, 1H); 4.44~4.40(m, 1H); 4.31~4.27( m, 1H); 4.10~4.00(m, 6H); 3.58~3.53(m, 1H); 3.47~3.40(m, 4H); 3.32(s, 3H); 3.31(s, 3H);
Embodiment 2
[0064] 5-Amino-3-(2,3-dihydroxypropylacetyl)-2,4,6-triiodoisophthalic acid (63.2 g, 100 mmol) was dissolved in 31.5 g DMAC and triethylamine ( 1.1g, 10mmol), cooled to below 20°C, slowly dropwise added methoxyacetyl chloride (43.4g, 400mmol), kept the temperature not exceeding 20°C during the dropwise addition, and slowly raised the temperature to 80°C, and kept the temperature for 1 Hours, HPLC detection monoamide content ≥ 95%. Distill the solution to dryness under reduced pressure, add 150ml mixed organic solvent, heat to dissolve, cool to crystallize, filter, wash with a small amount of mixed organic solvent to obtain 71.6g of the title compound as a white solid, HPLC purity ≥ 96%, yield 79.8%.
[0065] Step 1-a
Embodiment 3
[0067] 5-Methoxyacetamido-3-(2,3-dihydroxypropylacetyl)-2,4,6-triiodoisophthalic acid (70.4 g, 100mol) and methoxyacetic anhydride (81.0 g, 500 mmol), DMAP (0.63 g, 5.1 mmol) were mixed and heated to 100°C, stirred for 5 hours, and the monoamide content was ≥98% by HPLC. Distill the solution to dryness under reduced pressure, add 150ml of mixed organic solvent, heat to dissolve, cool to crystallize, filter, wash with a small amount of mixed organic solvent to obtain 72.3g of the title compound as a white solid, HPLC purity ≥ 98%, yield 83.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com