Bicyclol-tiopronin ester and preparation method and applications thereof
A technology of tiopronin ester and tiopronin, applied in the directions of active ingredients of heterocyclic compounds, production of bulk chemicals, digestive system, etc., can solve the problems of low oral bioavailability, inability to make injections, poor water solubility of bicyclic alcohols, etc. , to achieve low cost, reduce the degree of acute liver injury, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 bicyclic alcohol-tiopronin ester
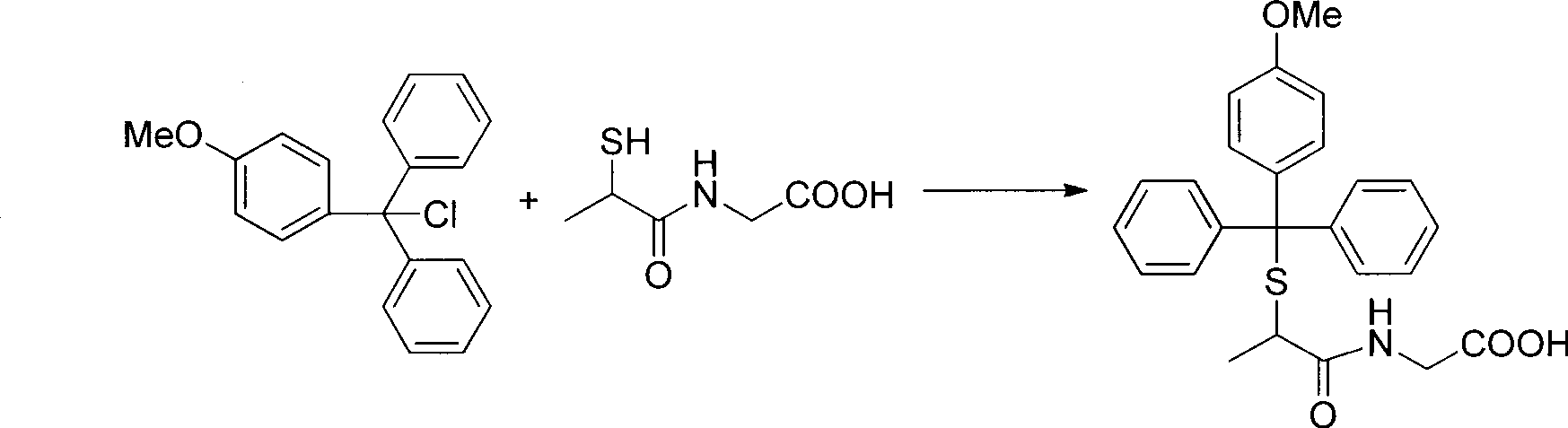
[0023] (1) Tiopronin sulfhydryl protection
[0024]
[0025] Dissolve 2.03g (32mmol) of tiopronin in 30mL of anhydrous N,N-dimethylformamide (DMF), and add 4.22g (13.7mmol) of monomethoxytrityl chloride (Mmt-Cl) at room temperature After 3 hours of reaction, TLC detected that the reaction was complete. The reaction liquid was evaporated to dryness, and the obtained solid was washed successively with ether, recrystallized from methanol, and dried to obtain 4.93 g of white powdery solid. (2) Esterification reaction
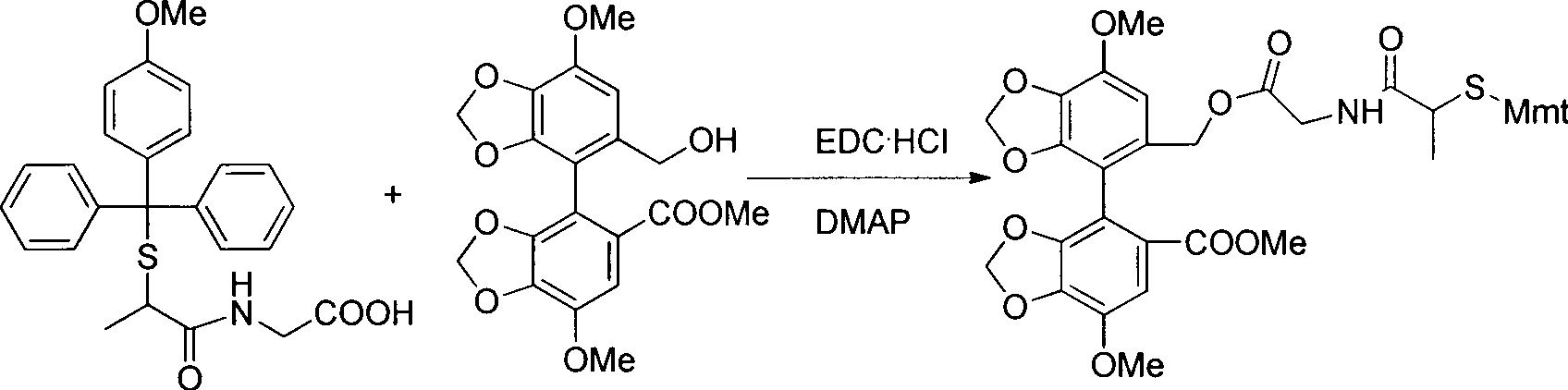
[0026]
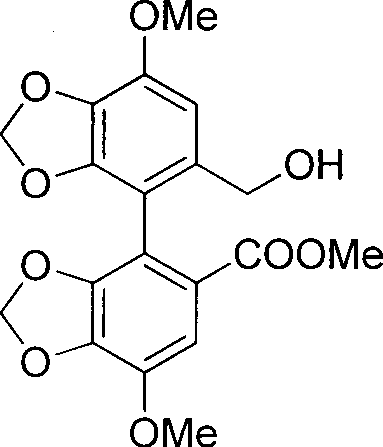
[0027] Dissolve 1.23 g (2.8 mmol) of the product obtained in step (1) in 20 mL of anhydrous dichloromethane (DCM), cool in an ice bath, add 4 mg of 4-dimethylaminopyridine (DMAP) (0.03 mmol) and 698 mg of 1-(3- Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl) (3.64mmol), stirred for 10min, added 911mg of bicyclic alcohol (2.3mmol), reacted for 2h. The reactio...
Embodiment 2
[0032] Example 2 Effect of bicyclol-tiopronin ester on rat D-galactosamine liver injury model
[0033] 1. Experimental materials
[0034] 1.1 Animals
[0035] SPF grade SD rats, weighing 180g-220g, male and female, were provided by the Comparative Medicine Center of Nanjing Military Region General Hospital. They were randomly divided into 4 groups, 10 rats in each group, namely (1) blank control group, (2) model control group, (3) bicyclol group, (4) bicyclol-tiopronin axate group.
[0036] 1.2 Drugs
[0037] The bicyclic alcohol-tiopronin ester of embodiment 1, bicyclic alcohol.
[0038] 1.3 Drug preparation, dosage and grouping
[0039] (1) blank control group;
[0040] (2) Model control group;
[0041] (3) Bicyclol group: administration of bicyclol at a dose of 6.75 mg / kg (according to the clinical human daily dosage of 75 mg and converted into rat dosage according to body surface area);
[0042] (4) Bicyclol-tiopronin ester group: Bicyclyl-tiopronin ester was admini...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com