Levetiracetam medicine composition and preparation method thereof
A kind of composition and medicine technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
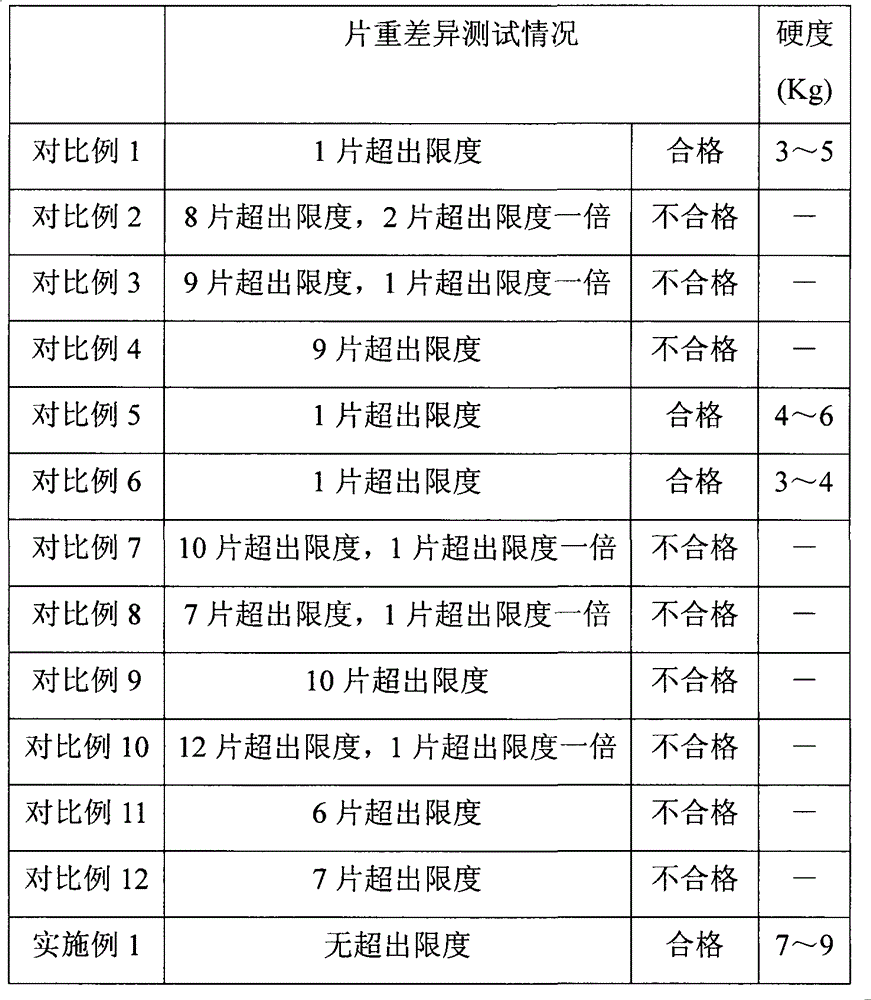
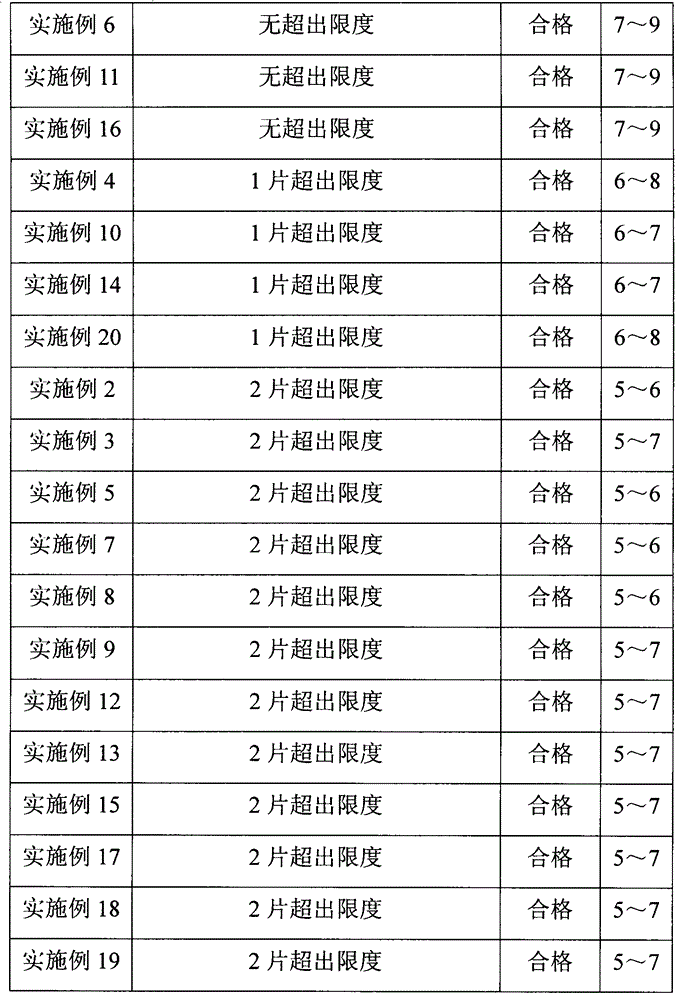
[0070] Example 1 to Example 20 and Comparative Examples 1, 5, and 6 were all tested according to the method for testing the dissolution rate of levetiracetam tablets stipulated in the "United States Pharmacopoeia". The test uses the paddle method, the speed is 50 rpm, the dissolution medium is water, the volume is 900mL, and the temperature is 37°C. The dissolution percentage is detected at 5min, 15min, 20min, 25min, and 30min respectively. Further testing its dissolution rate. Test result shows, the dissolution rate of embodiment 1 to embodiment 20 and comparative example 1,5,6 is all greater than 70% at 15min, meets " United States Pharmacopoeia " standard, wherein preferred prescription embodiment 4, embodiment 10, embodiment 14. The dissolution rate of Example 20 is greater than 92% at 15 minutes, and the dissolution rate of Example 1, Example 6, Example 11, and Example 16 are all greater than 96% at 15 minutes, and the effect is the best.
[0071] In summary, the prescri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com