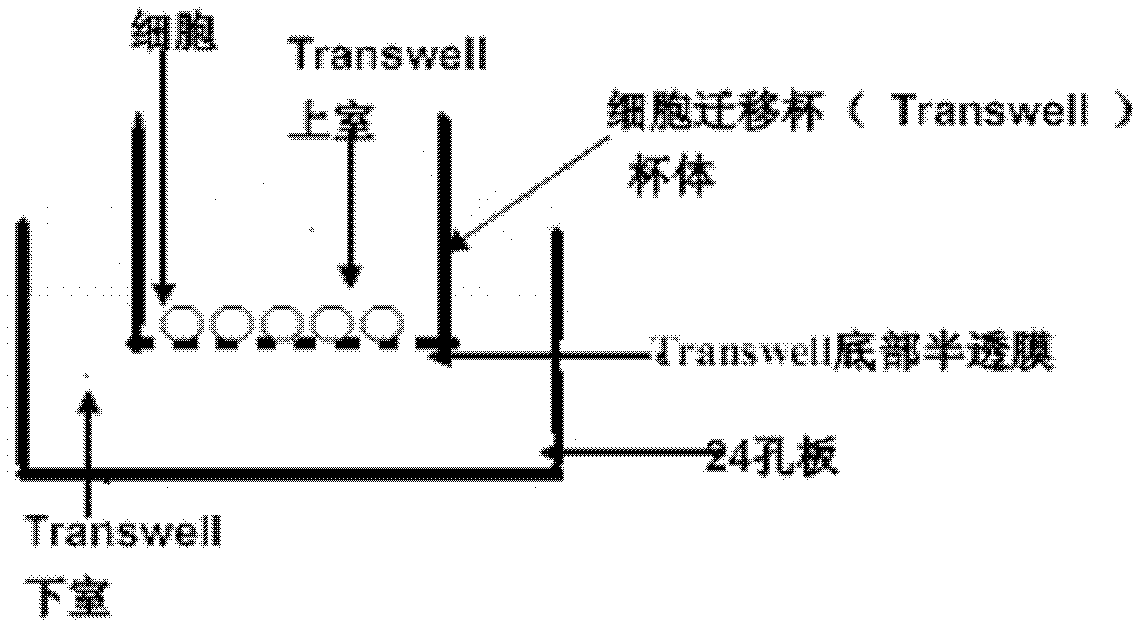
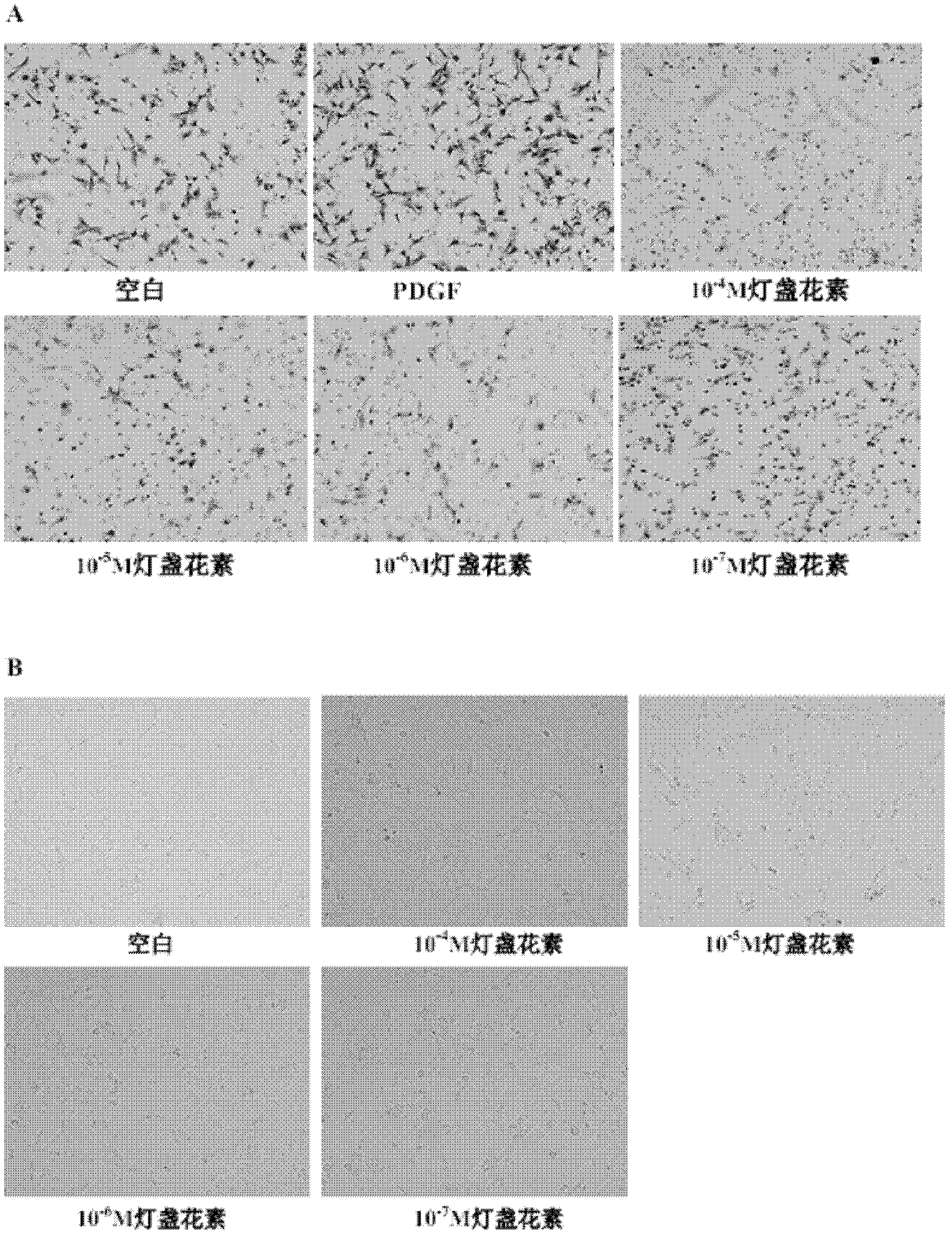
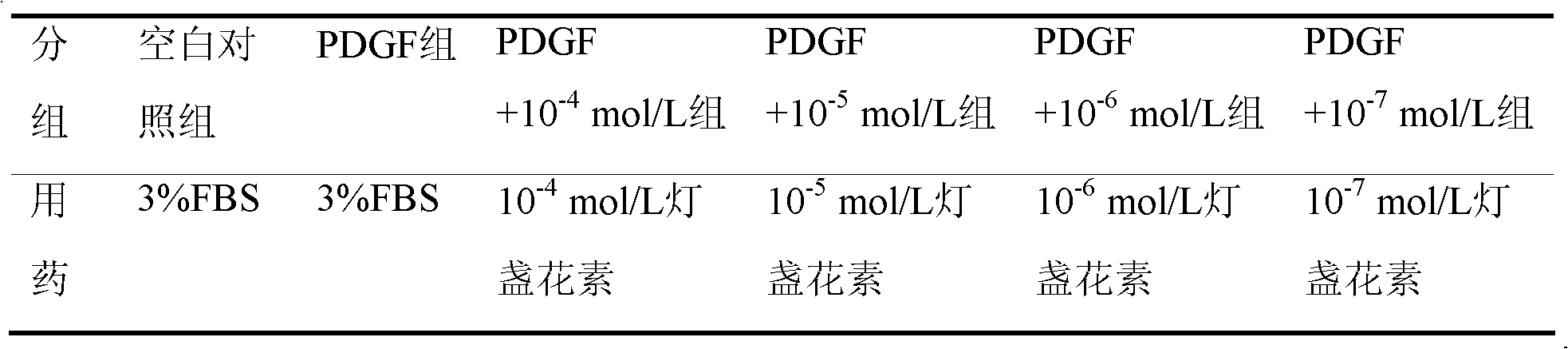
Application of breviscapine in hepatic stellate cell migration inhibition drug preparation
A technology of hepatic stellate cells and breviscapine, applied in the field of medicine, can solve the problems of hepatic stellate cell migration that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The scutellarin of the present invention is made into tablets according to methods known to those skilled in the art. Breviscapine tablets, each containing 20mg breviscapine, 15mg microcrystalline cellulose, 15mg micronized silica gel, 0.5mg magnesium stearate, and 8mg lactose. Usage: 2 tablets each time, 2-3 times a day, 5-6 days a week, every 3-4 weeks as a course of treatment.
Embodiment 2
[0067] The scutellarin of the present invention is made into capsules according to methods known to those skilled in the art. Breviscapine capsules, each capsule contains 20mg of breviscapine. Usage: 2 capsules each time, 2-3 times a day, 5-6 days a week, every 3-4 weeks as a course of treatment.
Embodiment 3
[0069] The scutellarin of the present invention is prepared into granules according to methods known to those skilled in the art. Breviscapine granules, each bag contains 20mg breviscapine, 15mg microcrystalline cellulose, 15mg micronized silica gel, 0.5mg stearic acid, and 8mg lactose. Usage: 2 bags each time, 2-3 times a day, 5-6 days a week, every 3-4 weeks as a course of treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com