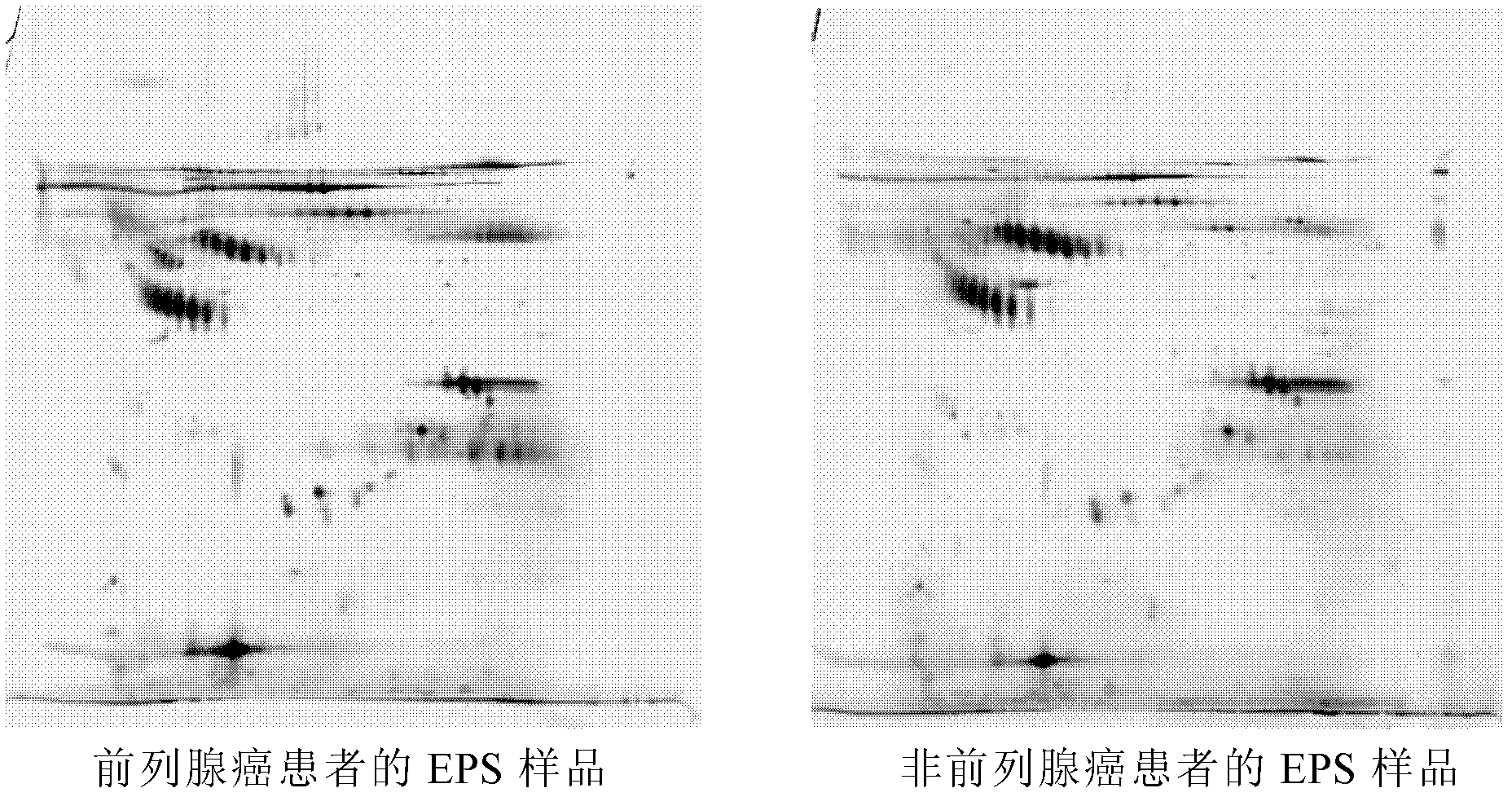
Prostatic cancer markers from prostatic secretion
A prostate cancer and prostatic fluid technology, applied in the fields of biotechnology and clinical diagnosis, can solve the problems of waste of medical resources and costs, ineffective results, low specificity and sensitivity of clinical diagnosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1, collection and processing of prostatic fluid
[0055] 1.1 Add 5-10ul, 300 times working solubility, 3 kinds of proteolytic enzyme inhibitor mixture (working solubility is as follows: Leupepetin 5ug / ml, Antipain 5ug / ml and Chymo statin 10ug / ml) to 1.5ml Eppendorf tube.
[0056] After aliquoting, store the tubes in a -20°C refrigerator for later use, or use them directly in the next steps.
[0057] 1.2 Use conventional clinical techniques to collect prostate fluid and drop it into the tube of 1.1 (about 50ul).
[0058] 1.3 Mix briefly (avoid Votexing). The following steps are all carried out at low temperature.
[0059] 1.4 centrifugation. 3000rpm / min, 5-10 minutes.
[0060] 1.5 Collect the supernatant and transfer it to another pre-cooled Eppendorf tube, this part is the prostate secretion (EPS). When transferring the liquid, do not disturb the sedimented fraction.
[0061] 1.7 All samples above should be stored in -80°C refrigerator.
[0062] 1.8 Ma...
Embodiment 2
[0063] Embodiment 2, the proteomics research method of sample
[0064] 2.1 EPS sample processing
[0065] 2.1.1 Quantitatively determine the protein concentration of each sample by conventional methods.
[0066] 2.1.2 Mix 20 EPS samples of prostate cancer patients and non-cancer patients of the same age in equal amounts (20ug protein / part), that is, the samples of the experimental group (30 samples of prostate cancer patients) and the control Group samples (mixture of 30 samples from non-cancer patients).
[0067] 2.1.2 Add trichloroacetic acid to the sample with a final concentration of 50% (weight / volume) to precipitate proteins and achieve the purpose of moderately purifying proteins. The samples were centrifuged at 5000 rpm / min for 5 minutes, and the supernatant was removed. Take the routine sample hydration buffer stored at -20°C from the refrigerator, and dissolve it at room temperature. Add a total volume of 250ul sample hydration buffer to each group of samples and...
Embodiment 3
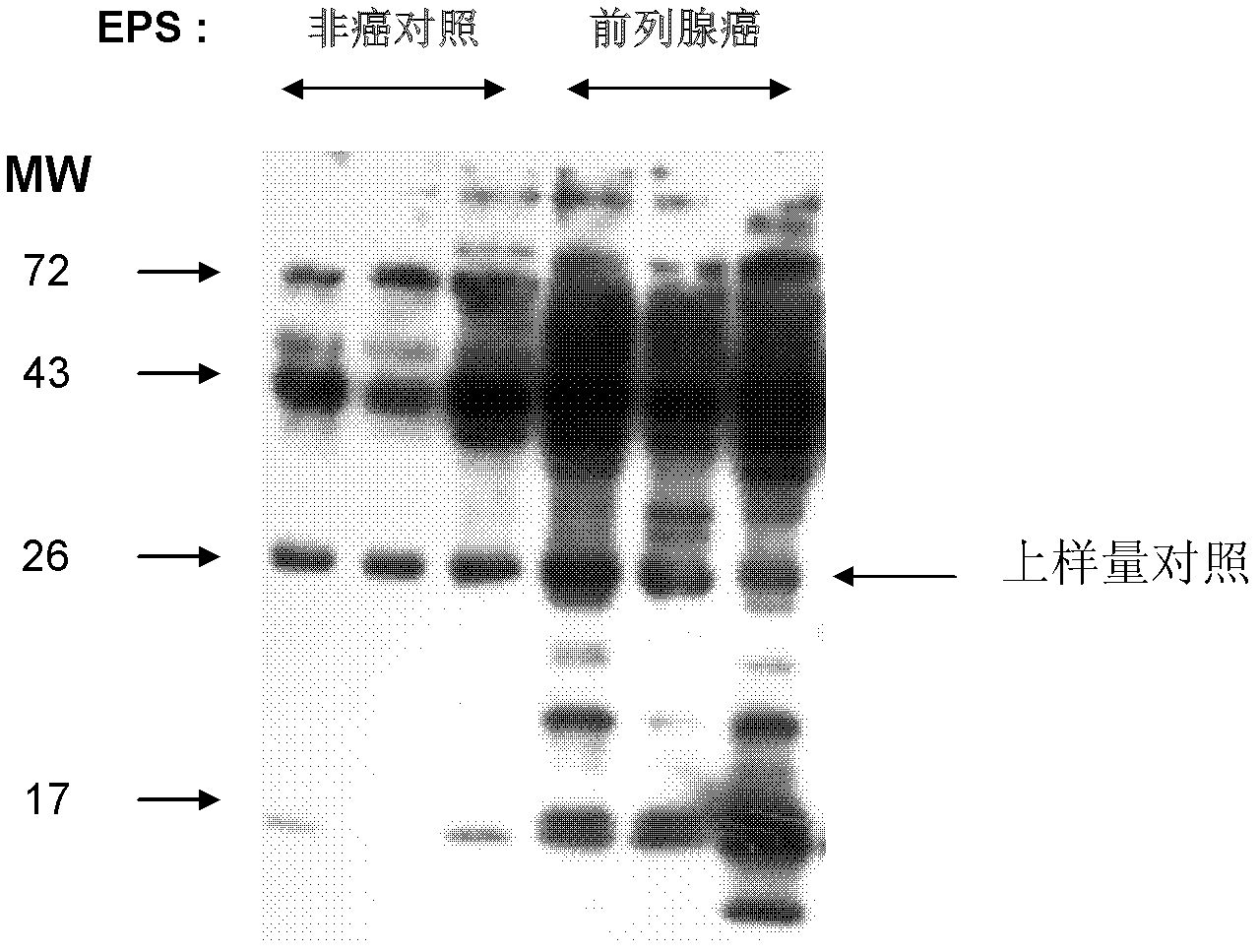
[0083] Example 3, Detection of Hepatoglobin Levels in Prostate Secreted Fluid of Prostate Cancer Patient Group and Non-cancer Control Group
[0084] Prostate cancer patient group and non-cancer control group were randomly divided into three copies of prostate secretion fluid, and 10 micrograms of protein in each copy were used for Western Blotting analysis. The rabbit polyclonal antibody (primary antibody) of Hepatoglobin is the product of AbCam Company (ab85846), and the dilution factor is 3000-5000 times when used. In addition, other details involved in the Western Blotting analysis of prostatic fluid are conventional techniques. figure 2 Shows the results of Western Blotting analysis of prostatic fluid.
[0085] According to literature reports, the full-length human Hepatoglobin protein consists of 406 amino acids. Among them, the leader peptide consists of 18 amino acids, and the alpha chain consists of 144 amino acids. The beta chain consists of 244 amino acids. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com