Compositions and methods for targeting type I interferon producing cells
A cell and compound technology, applied in chemical instruments and methods, drug combinations, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve problems such as being unsuitable for the treatment of chronic autoimmune diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
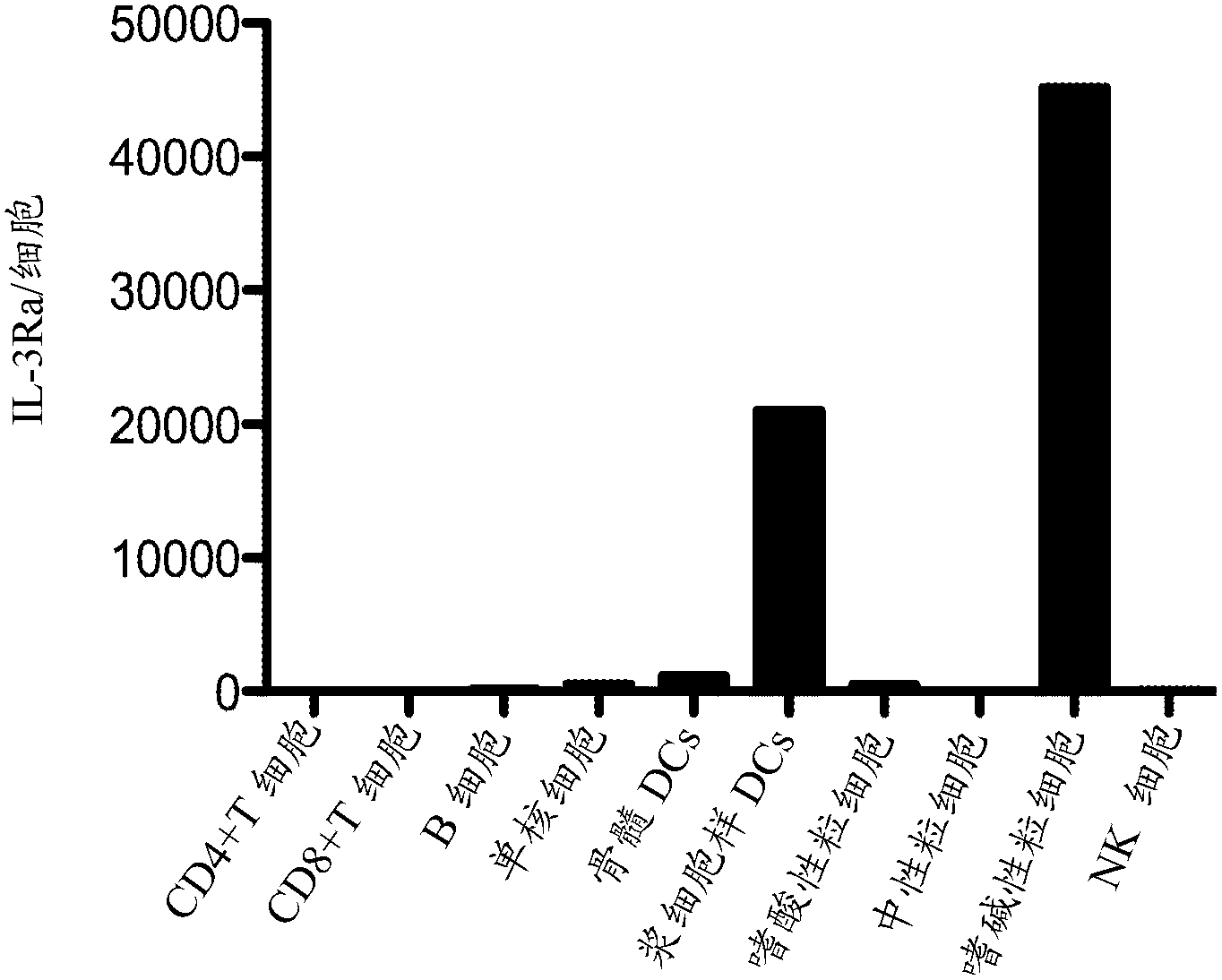
[0344] IL-3Rα expression
[0345] Use standard techniques to identify PBMC from humans, and isolate various cell lines using antibodies that bind to lineage-specific cell surface markers. Use Quantibrite TM Beads (BD Biosciences) to determine the number of IL-3Rα molecules per cell of each lineage. Such as figure 1 As shown, IL-3Rα is highly expressed on pDC and basophils, while expressed at low levels on other cell lineages tested. This restricted expression pattern makes IL-3Ra a useful target for antibodies designed to selectively eliminate pDC and basophils.
Embodiment 2
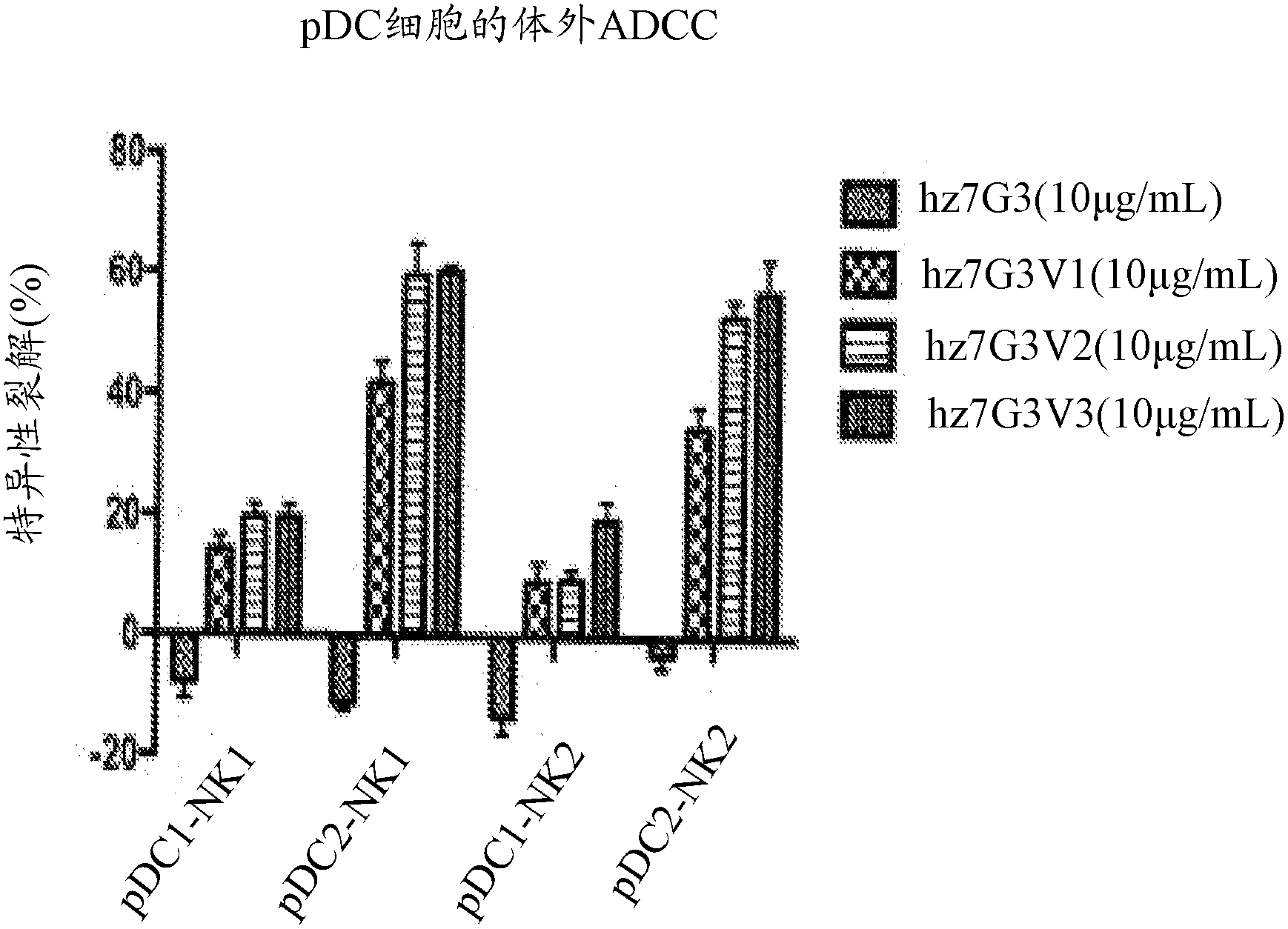
[0347] Anti-IL-3Rα mAb depletes human pDC in vitro
[0348] By Ficoll TM Isolation Isolate peripheral blood mononuclear cells (PBMC) from normal donors, and place the PBMC in the absence of antibodies (no antibodies), 10μg / ml anti-ch7G3, or 10μg / ml anti-hz7G3V3 at 37°C. Incubate in RPMI / 10%FCS for different times. In a 96-well U-bottom plate, routinely culture 1x10 in a volume of 200μL 6 Cells. Analysis of the number of plasmacytoid dendritic cells (pDC) and the number of basophils by flow cytometry (Tables 1 and 2, respectively). Identification of human pDC as negative for lineage markers by flow cytometry (CD20 - , CD3 - , CD14 - , CD19 - CD56 - ), HLA-DR positive, CD11c negative and IL-3Rα positive (see the gate control box in the flowchart). Identification of human basophils by flow cytometry as negative for lineage markers (CD20 - , CD3 - , CD14 - , CD19 - CD56 - ), IgE positive and IL-3Rα positive. The modified anti-IL-3Rα antibody (hz7G3V3) contained in the Fc domain c...
Embodiment 3
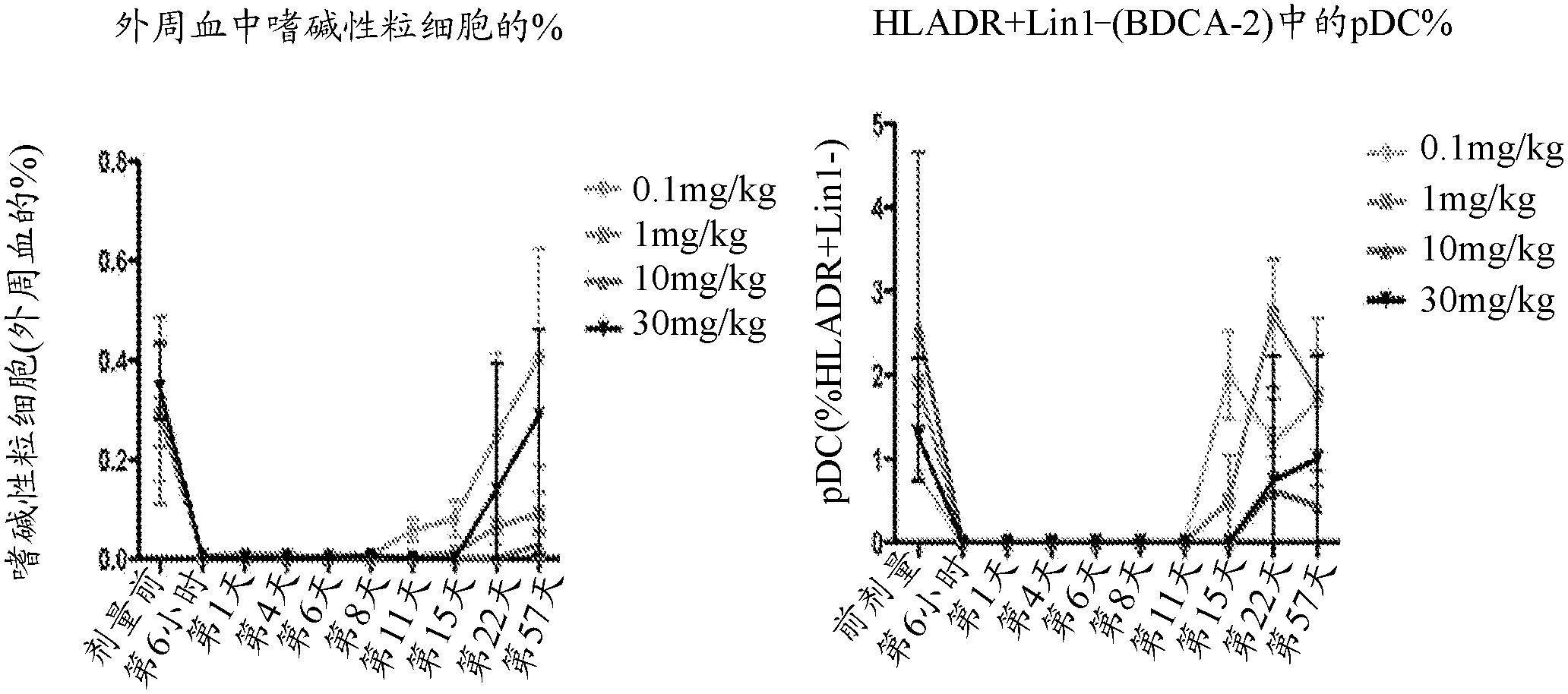
[0354] In vivo anti-IL-3Rα consumption of pDC and basophils in non-human primates
[0355] Non-GLP cynomolgus monkey non-human primate (NHP) research was conducted in the Australian National Primate Facility in accordance with its standard operating procedures. All operations and modifications were approved by the Institutional Animal Care and Use Committee (Institutional Animal Care and Use Committee). A single dose of neutralizing anti-IL-3Rα antibody (hz7G3V3) was given to young monkeys by intravenous infusion, which has a modification in the Fc domain to enhance the effector function of the antibody. Peripheral blood was collected at different time points and analyzed by flow cytometry for NHP basophils and pDC. NHP basophils were identified as positive for IgE+ / CD123 by flow cytometry. Identification of pDC as negative for lineage markers by flow cytometry (CD20 - , CD3 - , CD14 - , CD19 - , CD56 - ), HLA-DR positive, CD11c negative and IL-3Rα positive.
[0356] At all dose...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com