Hematopoietic stem cell cryopreserving method and protective agent
A hematopoietic stem cell and protective agent technology, applied in the field of hematopoietic stem cell cryopreservation, can solve the problems of restricting hematopoietic stem cell transplantation, cumbersome operation, high cost, etc., and achieve the effect of easy mastering and popularization, simple equipment and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The present invention is further described below in conjunction with specific embodiments, rather than limiting the present invention.
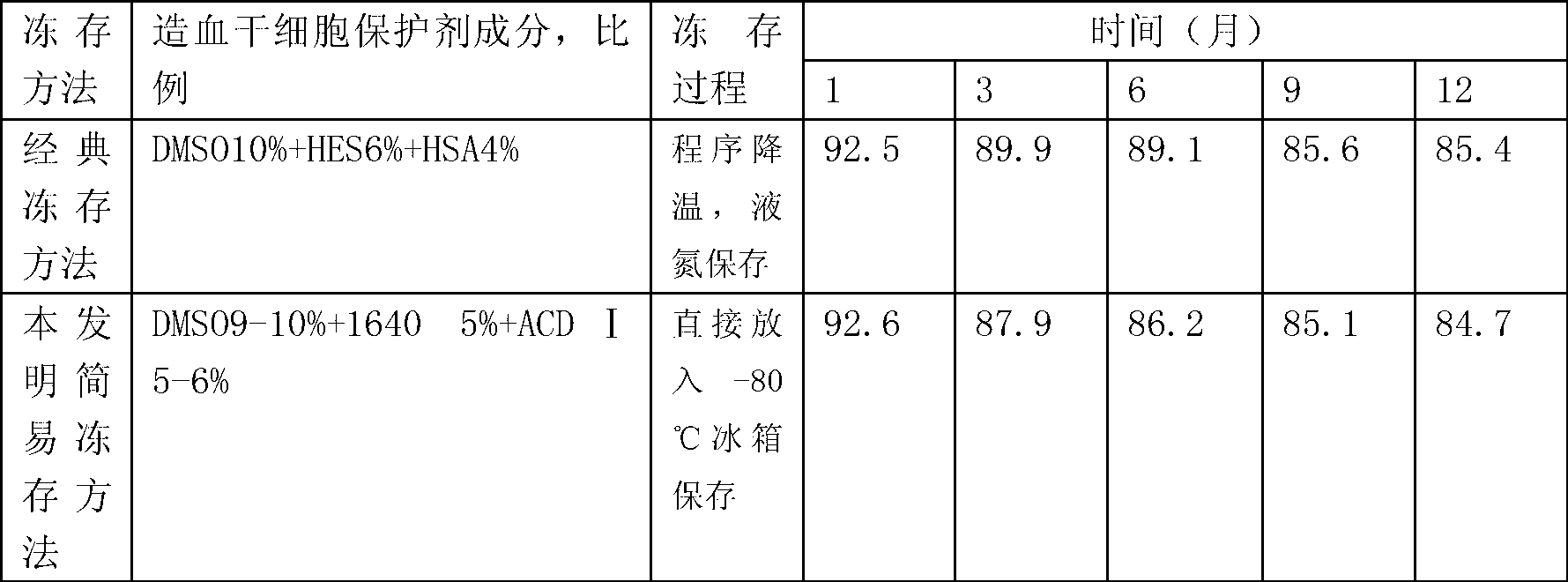
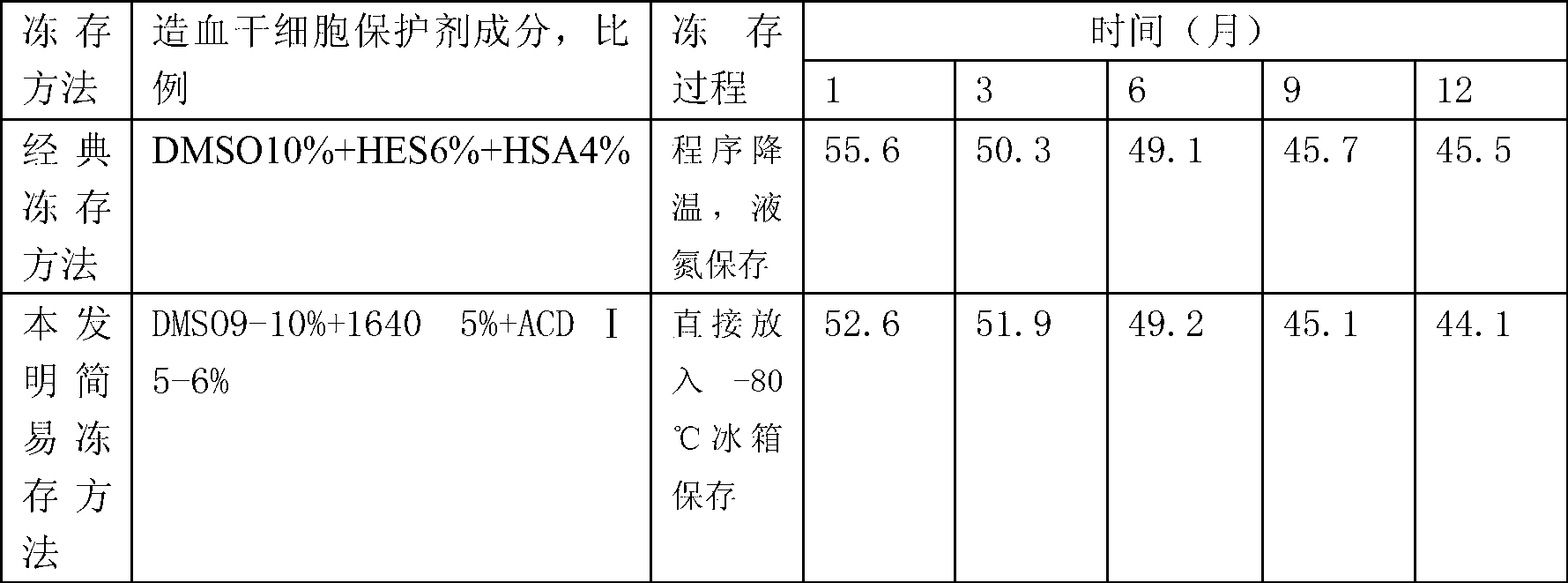
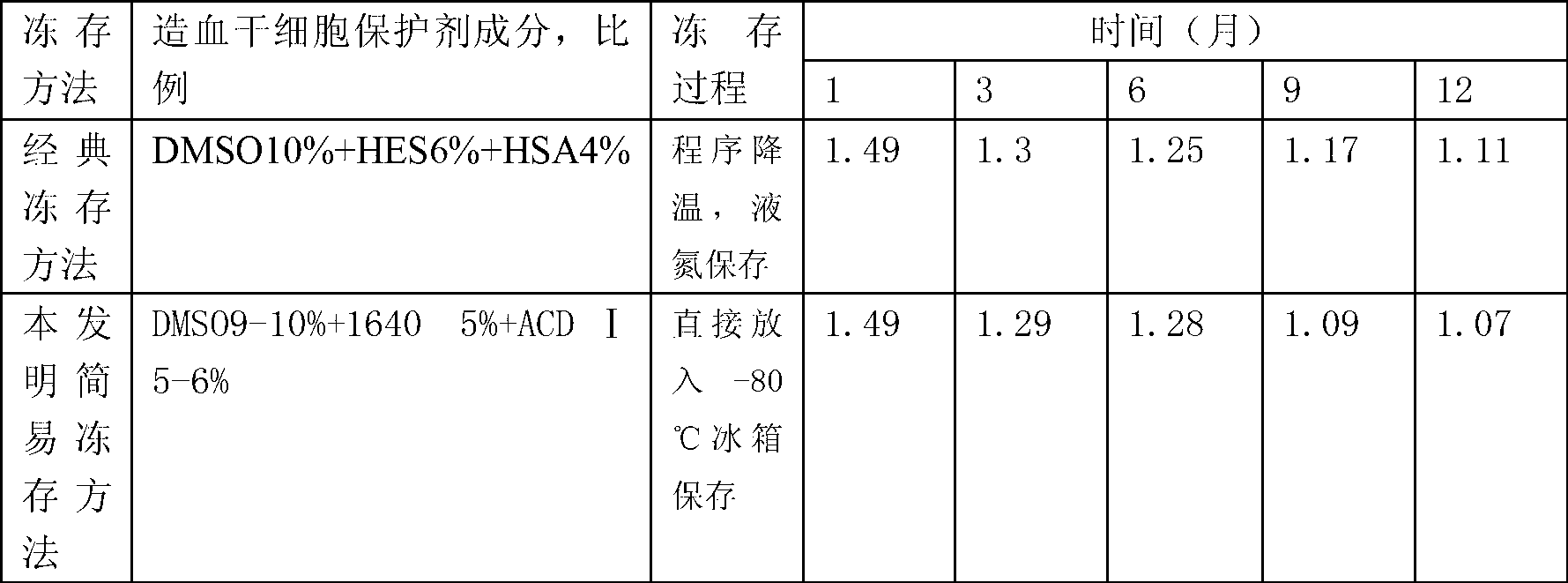
[0014] Transfer the sterile blood collection bag containing the hematopoietic stem cell suspension separated by the blood cell separator or the bone marrow fluid extracted by the operation to the aseptic operation room or the ultra-clean table. Choose DMSO, 1640 sterile medium and the blood preservation solution used by the blood cell separator Ⅰ (ACD Ⅰ) solution as a stem cell protective agent, the final ratio of the mixture of stem cell protective agent and hematopoietic stem cell suspension or surgically extracted bone marrow fluid is 9-10% DMSO, 1640 sterile medium 5%, and ACD Ⅰ solution 5- 6%, 79-81% of hematopoietic stem cell suspension or bone marrow fluid, in which hematopoietic stem cell suspension or bone marrow fluid does not need to be treated with red blood cells. First, the three components of the stem cell protectant, DMSO,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com