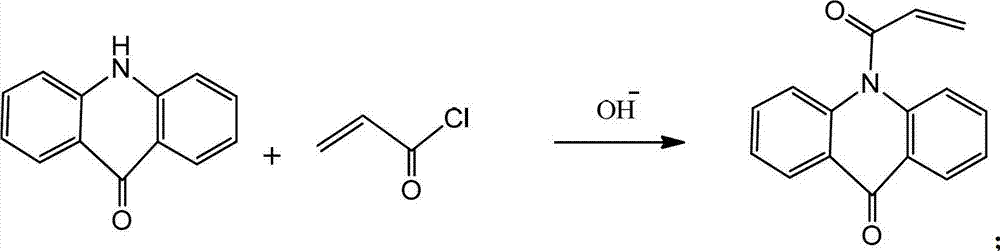
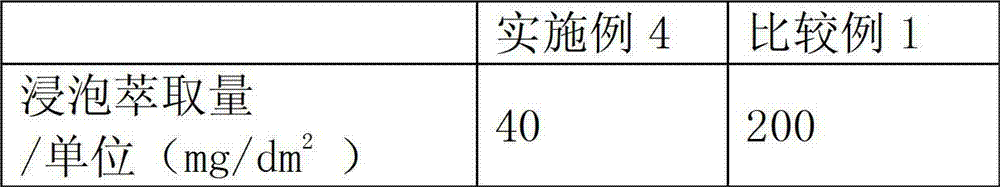
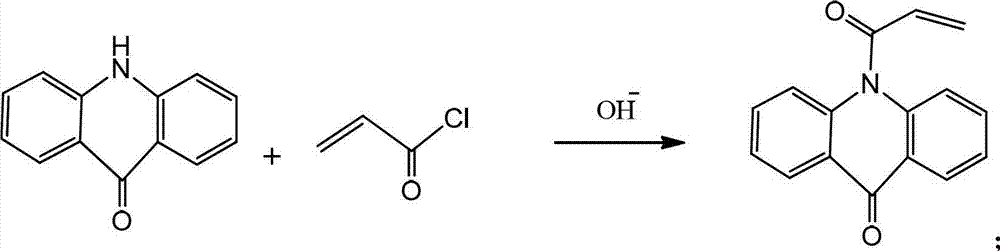
Polymerizable acridone photoinitiator and preparation method thereof
A technology of polymerizing photoinitiators and photoinitiators, which is applied in the field of photocuring, can solve problems such as potential safety hazards, odor migration, etc., and achieve the effect of simple preparation process and avoiding environmental and safety problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Put 19.7g of acridone, 11.1g of triethylamine, and 100mL of toluene into a 250ml four-neck flask equipped with stirring, and dropwise add acryloyl chloride at a temperature of 10°C. After about 30min, the dropwise addition is completed, and then continue to stir for 1h. To stop the reaction, add 50 mL of deionized water to the reaction bottle, stir for 10 min, pour the reaction solution into a separatory funnel for liquid separation, remove the lower water layer, and then extract 3 times with 50 mL of deionized water, and the organic layer was extracted with 10 g of deionized water. Dried with magnesium sulfate, filtered, and rotated under reduced pressure to remove toluene. The obtained solid was recrystallized with methanol and dried to obtain 22.8 g of a white solid product with a yield of 91%.
Embodiment 2
[0017] Put 19.7g of acridone, 15g of triethylamine, and 100mL of dichloromethane into a 250ml four-necked flask equipped with stirring, and dropwise add acryloyl chloride at a temperature of 10°C. After about 30min, the dropwise addition is completed, and then continue to stir for 1h. , to stop the reaction, add 50mL deionized water to the reaction bottle, stir for 10min, pour the reaction solution into a separatory funnel to separate the liquid, separate the water layer, and then extract 3 times with 50mL deionized water respectively, and the organic layer is extracted with 10g deionized water Dried over magnesium sulfate, filtered, and rotated under reduced pressure to remove the solvent. The obtained solid was recrystallized with methanol and dried to obtain 23.7 g of a white solid product with a yield of 95%.
Embodiment 3
[0019] Drop into 19.7g acridone, 11.7gNa 2 CO 3 , 100mL of dichloromethane, keep the temperature at 10°C and add acryloyl chloride dropwise, the dropwise addition is completed in about 30min, then continue to stir for 1h, stop the reaction, add 50mL of deionized water into the reaction bottle, stir for 10min, pour the reaction solution into Separate the liquid in a separatory funnel, separate the water layer, and then extract three times with 50 mL deionized water respectively, dry the organic layer with 10 g colorless magnesium sulfate, filter, and rotate under reduced pressure to remove the solvent. The obtained solid is recrystallized with methanol, baked Dry to obtain 17.9 g of white solid product, yield 72%.
[0020] The product obtained in Examples 1, 2, and 3 was tested as the target product through proton nuclear magnetic resonance spectroscopy.
[0021] 1 H-NMR (CDCl 3 , 500MHz): δ5.638 (2H, d, =CH 2 ), 6.468~1.527 (1H, m, -CH=), 7.285~8.042 (8H, m, benzene ring)...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap