Preparation method for oral hypoglycemic recombinant human proinsulin
A technology for recombinant human insulin and human insulin, applied in the direction of insulin, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of complex separation and purification process of human proinsulin, and achieve simple and rapid preparation method and separation process, reduce Negative effects and mental distress, overcoming the effects of complex separation and purification processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Cloning, sequencing and identification of human proinsulin hpi gene
[0038] The gene of human proinsulin HPI was amplified from the plasmid pDNR-LIB (Protein Group, Inc.) purchased by the company by PCR.
[0039]The PCR amplification primers of human proinsulin hpi gene fragment are:
[0040] hpi-F: CATGCCATGGCTATGGCCCTGTGGATGCG (SEQ ID NO. 1)
[0041] hpi-R: CGAGCTCCTAGTTGCAGTAGTTTCCAGCTG (SEQ ID NO. 2)
[0042] The PCR reaction system was carried out strictly according to the instruction manual of TaKaRa LA Taq. The 20 μL reaction system contained 0.2 μL of TaKaRa LA Taq, 10×LA PCR Buffer Ⅱ (Mg 2+ Plus) 2μL, dNTP Mixture (2.5mM each) 3.2μL, template DNA 100ng, upstream and downstream primers (20μM) 0.4μL, and finally add sterilized ultrapure water to make up to 20μL. The PCR conditions are: denaturation at 94°C for 5 minutes, 1 minute at 94°C, 40s at 56°C, and 40s at 72°C, 30 cycles. The size of the PCR product is 352bp, including the coding sequence o...
Embodiment 2
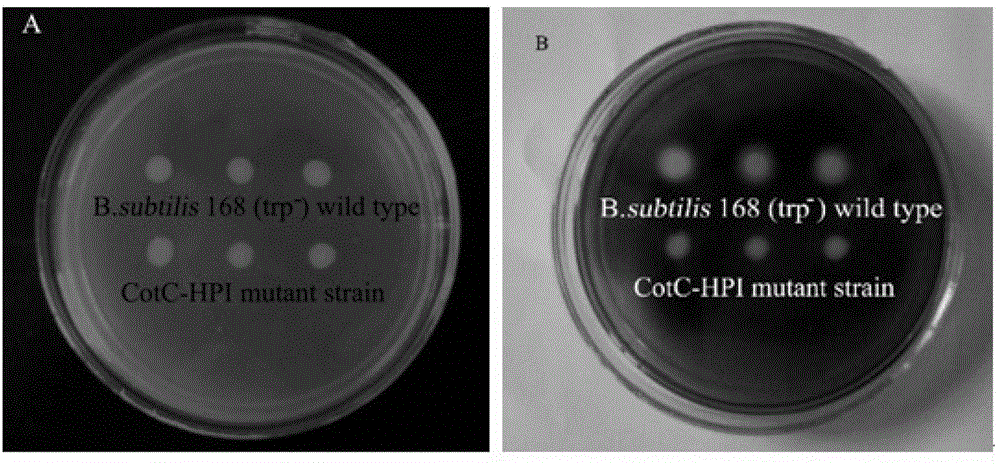
[0044] Example 2: Preparation and application of Bacillus subtilis recombinant spores displaying HPI on the surface of the carrier using CotC
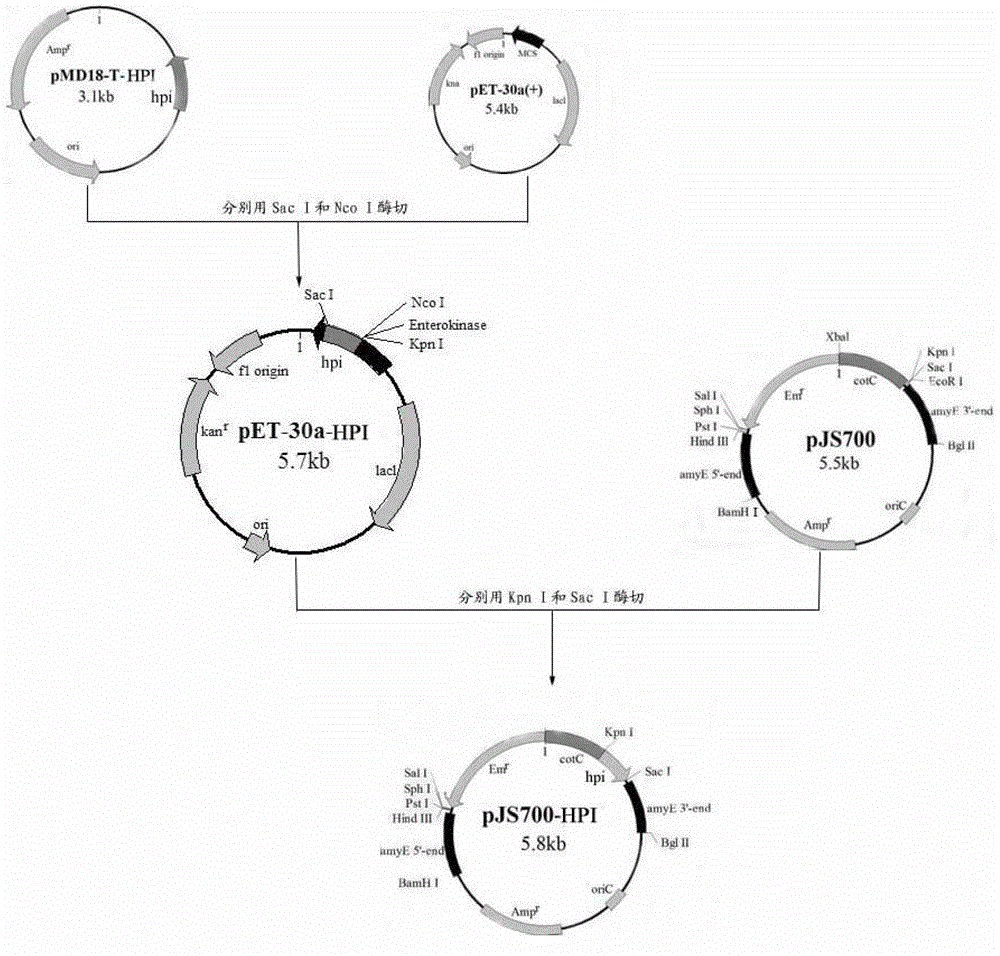
[0045] 1. Construction of recombinant integrative plasmid pJS700-HPI
[0046] Plasmid pJS700 (Li Qian. Study on recombinant spores of Bacillus subtilis displaying WSSV envelope proteins Vp19 and Vp28 on the surface of CotX [D]. Zhenjiang, Jiangsu: Jiangsu University, 2010:36-38) was provided by Ningde, School of Environment, Jiangsu University Donated by Associate Professor Gang, the size of the plasmid is about 5.5kb. In the pJS700 plasmid, amyE5'-end and amyE3'-end respectively represent the 5' end and 3' end of the coding sequence of the amylase gene amyE (Gene Bank sequence number: NP_388186), which are integrated into Bacillus subtilis 168 (trp - ) in the amylase gene of the chromosome. Amps r ,Em r Represent ampicillin resistance gene and erythromycin resistance gene respectively, in Escherichia coli and Bacillus subtilis 168...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com