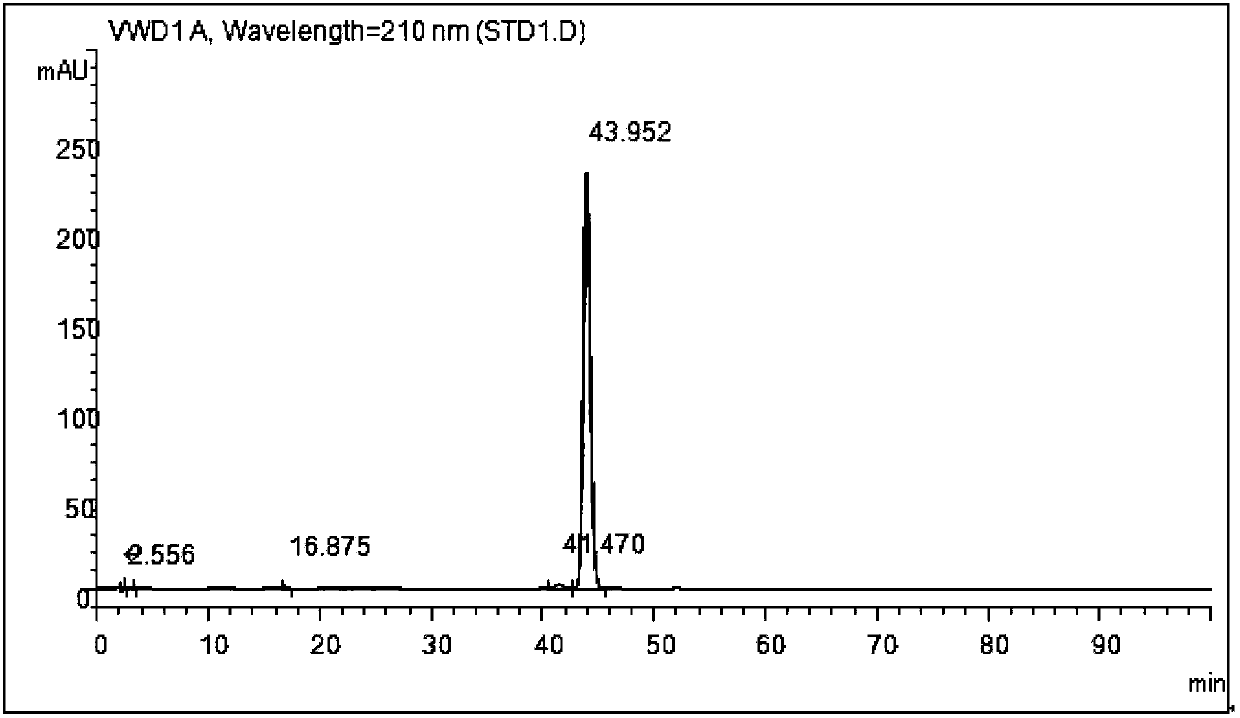
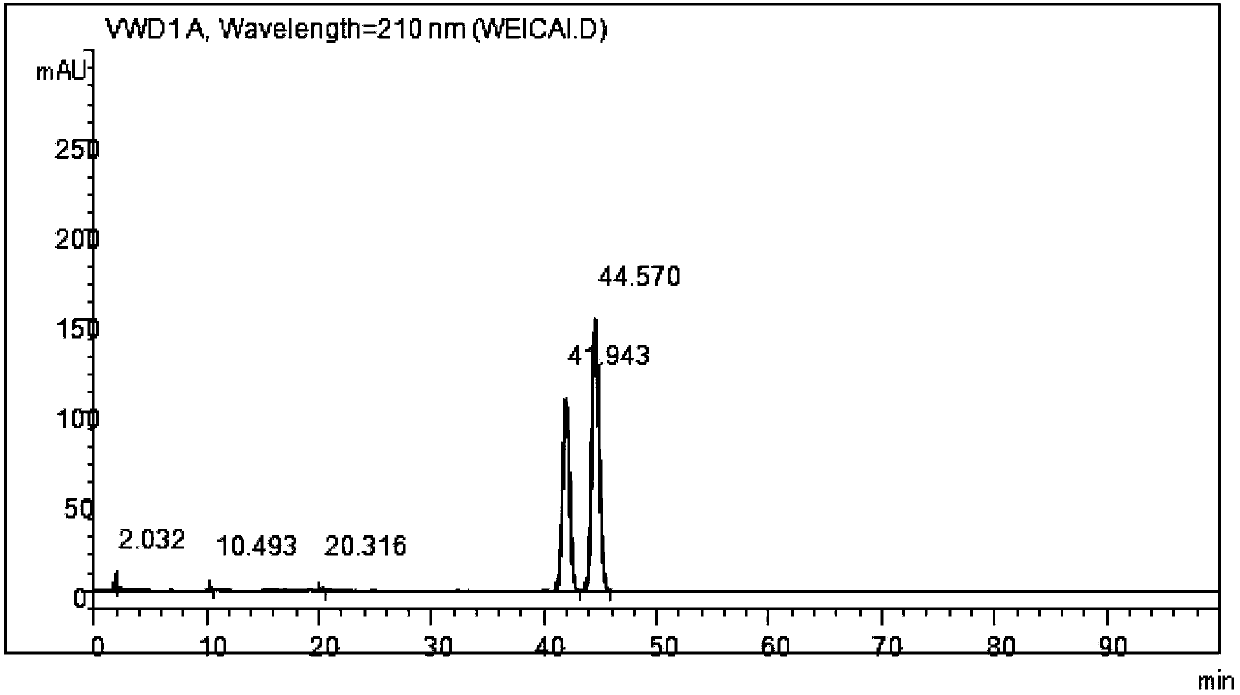
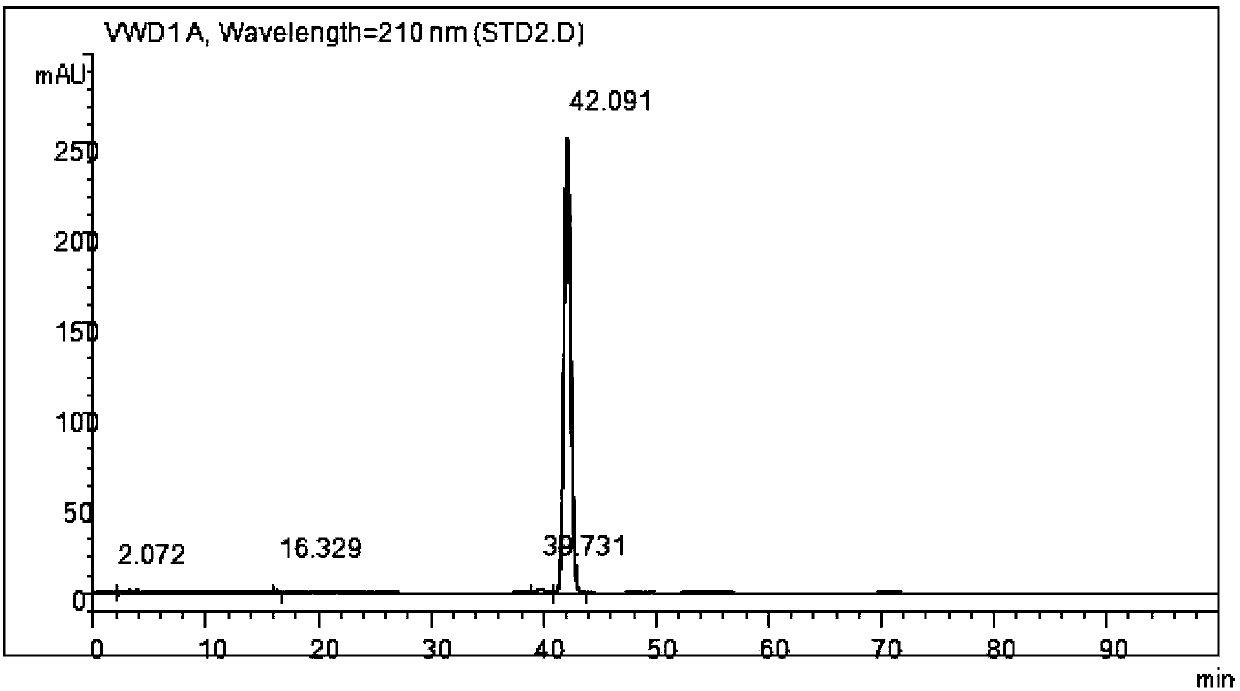
Process for synthesizing and purifying teprenone
A teprenone and process technology, applied in the field of drug synthesis and purification technology, can solve the problems of not specifically listing teprenone related content, many synthesis steps, difficult industrialization and the like, and achieves increased solubility and Catalytic ability, high reaction conversion rate, easy control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Synthesis of teprenone: 1000 grams of (6E, 10E)-geranyl linalool (the (6E, 10E)-geranyl linalool is (2E, 6E, 10E)-geranyl linalool and ( 2Z, 6E, 10E)-a mixture of two geometric isomers of geranyl linalool with a molar ratio of 3:2), 80 grams of aluminum isopropoxide and 125 grams of isopropanol were added to a 5-liter three-necked flask and heated and stirred to 50°C, keep stirring during the heating process until it dissolves completely, then add 800 grams of methyl acetoacetate, raise the temperature to 160°C at a speed of 3° / min, carry out Carroll rearrangement reaction, control the reaction time for 12 hours, use the above thin layer Qualitative detection by chromatography, there is no color in the product chromatogram corresponding to the chromatographic spot of (6E,10E)-geranyl linalool reference substance, the reaction has been completed, then the temperature is lowered to 40 degrees, and the concentration and recovery of lower alcohols is started under...
Embodiment 2
[0044] Example 2: Synthesis of teprenone: 1000 grams of (6E, 10E)-geranyl linalool (the (6E, 10E)-geranyl linalool is (2E, 6E, 10E)-geranyl linalool and ( 2Z, 6E, 10E) - a mixture of two geometric isomers of geranyl linalool with a molar ratio of 3:2), 80 grams of aluminum isopropoxide and 140 grams of isopropanol were added to a 5-liter three-necked flask and heated and stirred To 45 degrees, the heating process is constantly stirred until the dissolution is complete, then add 1100 grams of sec-butyl acetoacetate, and heat up to 150 degrees at a speed of 3 degrees / minute to carry out the Carroll rearrangement reaction. The control reaction time is 14 hours. Qualitative detection by layer chromatography, there is no color in the product chromatogram corresponding to the chromatographic spot of the (6E,10E)-geranyllinalool reference substance, and the reaction has been completed; then the temperature is lowered to 40 degrees, and the concentration and recovery of the lower grade...
Embodiment 3
[0046] Example 3: Synthesis of teprenone: 1000 grams of (6E, 10E)-geranyl linalool (the (6E, 10E)-geranyl linalool is (2E, 6E, 10E)-geranyl linalool and ( 2Z, 6E, 10E) - a mixture of two geometric isomers of geranyl linalool with a molar ratio of 3:2), 90 grams of aluminum sec-butoxide and 165 grams of sec-butanol were added to a 5-liter three-necked flask and heated and stirred to 45°C, keep stirring during the heating process until it dissolves completely, then add 1000 grams of ethyl acetoacetate, raise the temperature to 160°C at a rate of 3° / min, carry out Carroll rearrangement reaction, control the reaction time for 10 hours, use the above thin layer Qualitative detection by chromatography, there is no color in the product chromatogram corresponding to the chromatographic spot of (6E,10E)-geranyl linalool reference substance, the reaction has been completed, then the temperature is lowered to 40 degrees, and the concentration and recovery of lower alcohols is started unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com