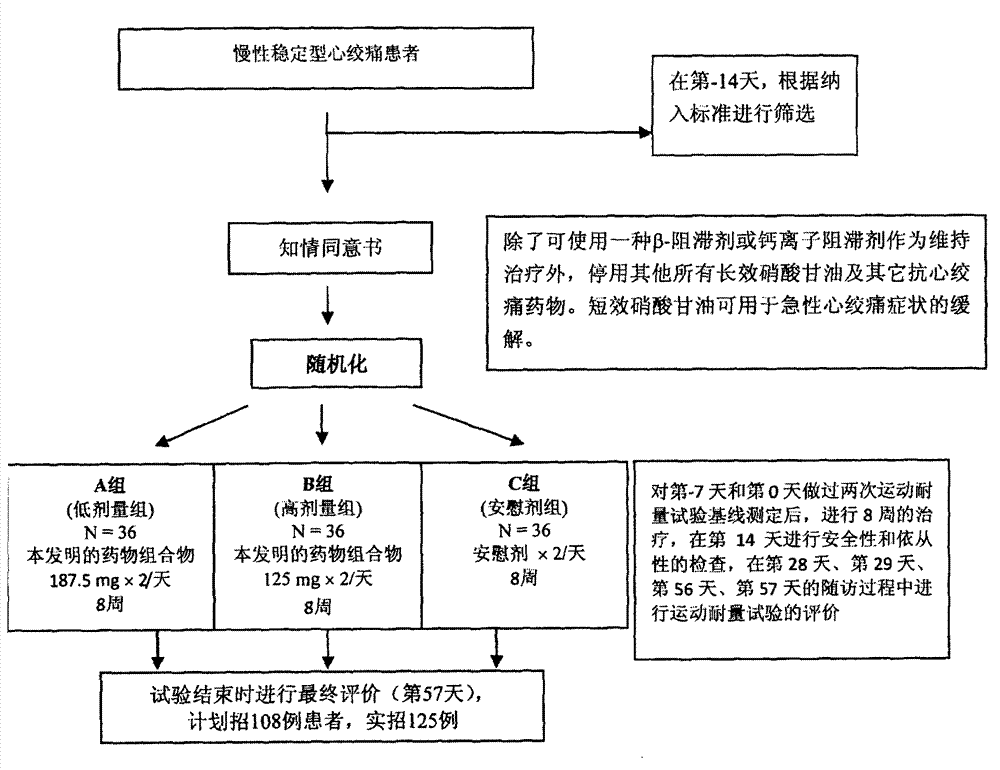
Application of Danshen composition in preparation of medicine for secondary prevention of coronary heart disease
一种二级预防、组合物的技术,应用在药物组合、心血管系统疾病、羟基化合物有效成分等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0187] Recipe dosage
[0188] Danshen 45.0g Panax notoginseng 47.0g Borneol 0.1g
[0189] Excipient polyethylene glycol-6000 18g
[0190] Make 1000 drop pills
[0191] Extraction of Danshen and Panax notoginseng
[0192] Take coarsely crushed Danshen medicinal materials (45.0g) and Panax notoginseng medicinal materials (47.0g) into the extraction tank, add 5 times the amount of water of the above crude drugs, decoct for 2 hours, filter, add 4 times the amount of water to the filter residue, and decoct The second extraction was carried out in 1 hour, filtered, the filter residue was discarded, and the filtrates were combined. The filtrate is concentrated under reduced pressure to a ratio of 1: 0.9-1.1 between the volume of the medicinal solution (L) and the weight (Kg) of the medicinal material, and slowly adds 95% (v / v) ethanol to make the alcoholic concentration of the medicinal solution at 69-71% ( v / v), let stand for 12 hours. The supernatant of the medicinal solution ...
preparation Embodiment 2
[0198] Take 70.0g Salvia miltiorrhiza and 13.7g Panax notoginseng, crush them, put them into an extraction tank, add 5 times the amount of water of the crude drug above, extract for 2 hours, filter, and collect the primary filtrate. Add 4 times the amount of water to the dregs, decoct for 1 hour, filter, mix with the filtrate extracted for the first time, and concentrate under reduced pressure until the ratio of solution volume (L) to crude drug mass (Kg) is 1:0.9-1.1. Add 95% (v / v) ethanol until the alcohol content reaches 69-71% (v / v), let stand for 12 hours, and filter. The filtrate is concentrated into an extract with a relative density of 1.32-1.40.
[0199] Mix the above extract with 0.8g borneol and 15.5g polyethylene glycol 6000, heat at 85°C, mix for 30 minutes, transfer to the dropping pill machine, keep warm at 80°C, drop into liquid paraffin at 7°C, take out the dropping pills, remove Liquid paraffin, that is.
[0200] Dropping pills are reddish-brown pills, unif...
preparation Embodiment 3
[0202] Take 96.0g Danshen, 1.0g Panax notoginseng, 3.0g borneol and 20g polyethylene glycol 6000 respectively. The extraction method was the same as that in Preparation Example 1; except for the following parameters, the temperature of the dropping pill machine was 64° C., and the temperature of the liquid paraffin was 0° C., and the method in Preparation Example 1 was used to prepare the dropping pills.
[0203] Dropping pills are reddish-brown pills, uniform in size, smooth, fragrant and slightly bitter. The pellet weight was 25mg±15%, and the diameter was 3.34±15%mm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com