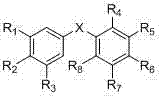
Diphenyl selenide, diphenyl selenoxide, diphenyl selenone compounds and uses thereof
A kind of technology of diphenylselenyl sulfoxide and diphenylselenide, which is applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
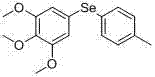
[0126] Example 1: Preparation of 3, 4, 5-trimethoxyphenyl-4-methylphenylselenide (compound 1)
[0127] 1 g of 3, 4, 5-trimethoxydiselenide was dissolved in anhydrous THF, and 0.77 g of lithium aluminum tetrahydrogen was added to react for 1 hour to obtain 3, 4, 5-trimethoxydiselenol. Add 0.25 g of p-methoxyaniline to another reaction flask, add 0.5 ml of concentrated hydrochloric acid, stir to dissolve, add 0.14 g of sodium nitrite at 0 degrees Celsius, and react for 30 minutes. Afterwards, 30 ml of water and 3 g of potassium carbonate were added to the prepared benzoselenol solution at 0 degrees Celsius, and the prepared diazonium salt solution was slowly added dropwise at 0 degrees Celsius. After the dropwise addition was completed, the reaction was carried out for 2 hours. Afterwards, the reaction solution was acidified and extracted with ethyl acetate, the organic layer was washed with saturated aqueous sodium chloride solution, the organic layer was separated and dried...
Embodiment 2
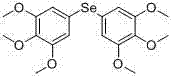
[0128] Example 2: Preparation of bis-3, 4, 5-trimethoxyphenyl selenide (compound 2)
[0129] Compound 2 was prepared in the same manner as in Example 1 except that corresponding raw materials were used, and the yield was 80%. ESI m / z 437.1[M+Na] +
Embodiment 3
[0130] Example 3: Preparation of 3, 4, 5-trimethoxyphenyl-p-chlorophenyl selenide (compound 3)
[0131] Compound 3 was prepared in the same manner as in Example 1 except that the corresponding raw materials were used, and the yield was 86%. 1 H-NMR (300M, CDCl 3 ), δ7.33(2H, d, J=8.62), 7.23(2H, d, J=8.62), 6.74(2H, s), 3.85(3H, s), 3.81(6H, s), ESI m / z 359.2[M+H] + , 381.2[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com