Artificially designed HIV (human immunodeficiency virus)-infection-resisting polypeptide, composition and application
A derivative, L-type technology, applied in the treatment or prevention of related diseases caused by HIV infection, acquired immunodeficiency syndrome, non-physiologically toxic salt, anti-HIV infection polypeptide field, can solve the problem of low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Embodiment 1: the preparation of compound 6
[0143] A standard Fmoc solid-phase peptide synthesis method was used. All peptide sequences are amidated at the C-terminus and acetylated at the N-terminus. Rink Amide resin is selected, the amino acid is in L configuration, and the peptide chain is extended from the C-terminus to the N-terminus. The condensing agent is HBTU / HOBt / DIEA. The deprotecting agent is piperidine / DMF solution. The lysing agent is trifluoroacetic acid (TFA), and the crude peptide is dissolved in water and then freeze-dried for storage. Separation and purification are carried out by medium-pressure liquid chromatography Flash or high-pressure liquid chromatography (HPLC), and the pure peptide content is >95%. Matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS) confirmed the molecular weight of the peptide sequence.
[0144] Use CEM automatic microwave peptide synthesizer for peptide synthesis, the synthesis conditions...
Embodiment 2-68
[0155] Embodiment 2-68: Preparation of Compound 1-5, 7-68
[0156] Compounds No. 1-No. 5, No. 7-No. 68 were synthesized according to the method of Example (1), and the amino acids were all in L configuration, but the corresponding amino acid residues were replaced.
Embodiment 69
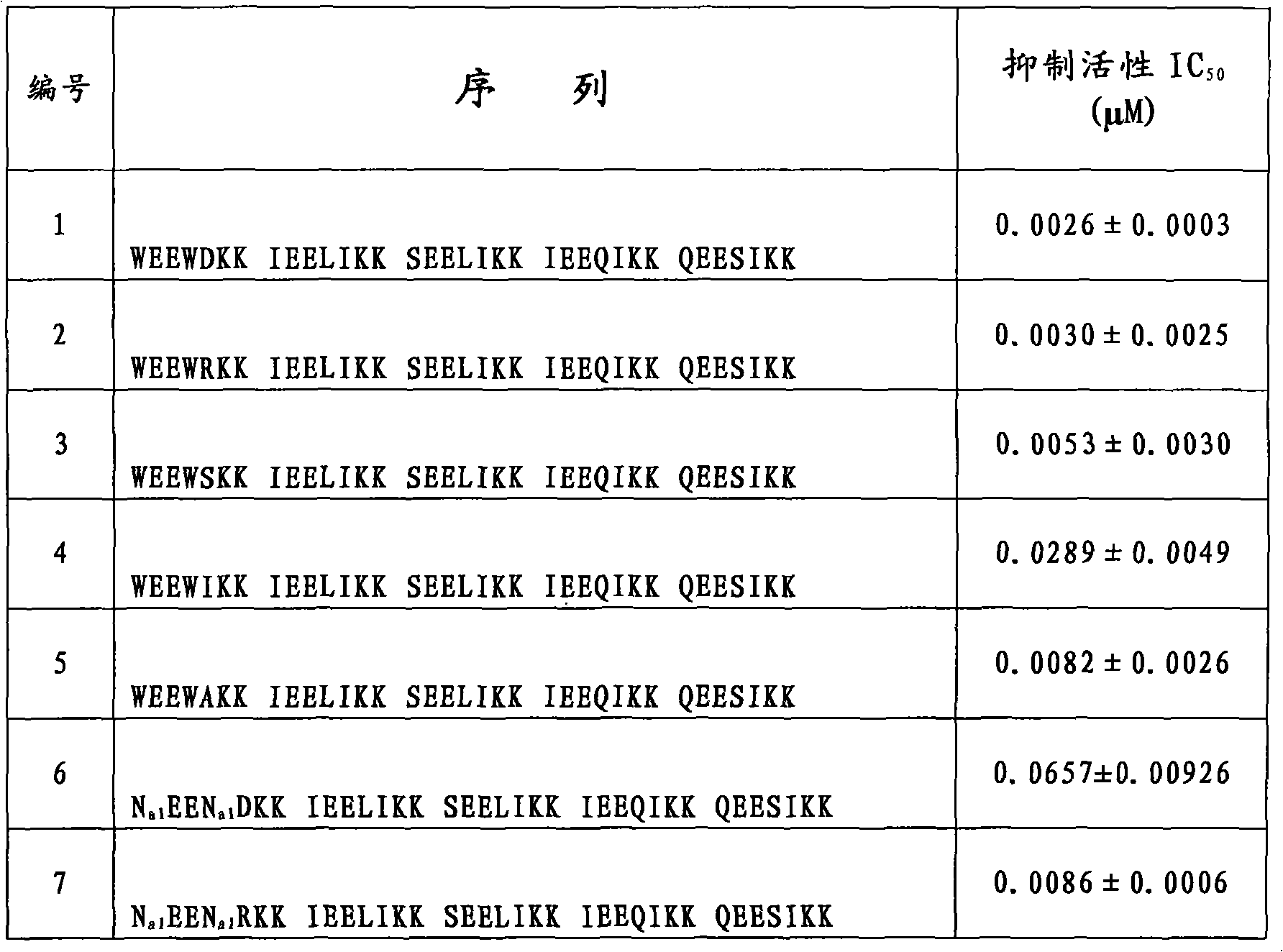
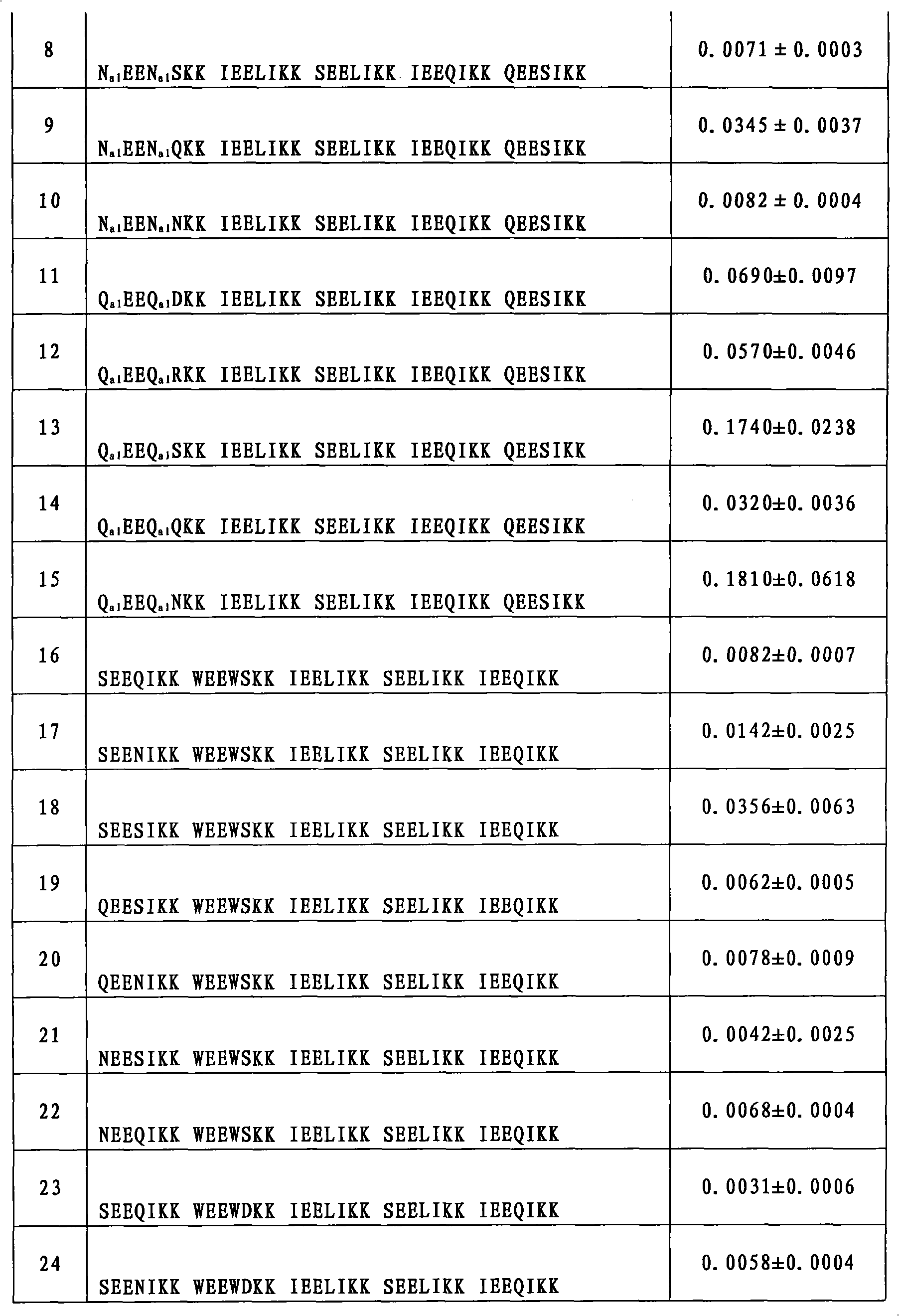
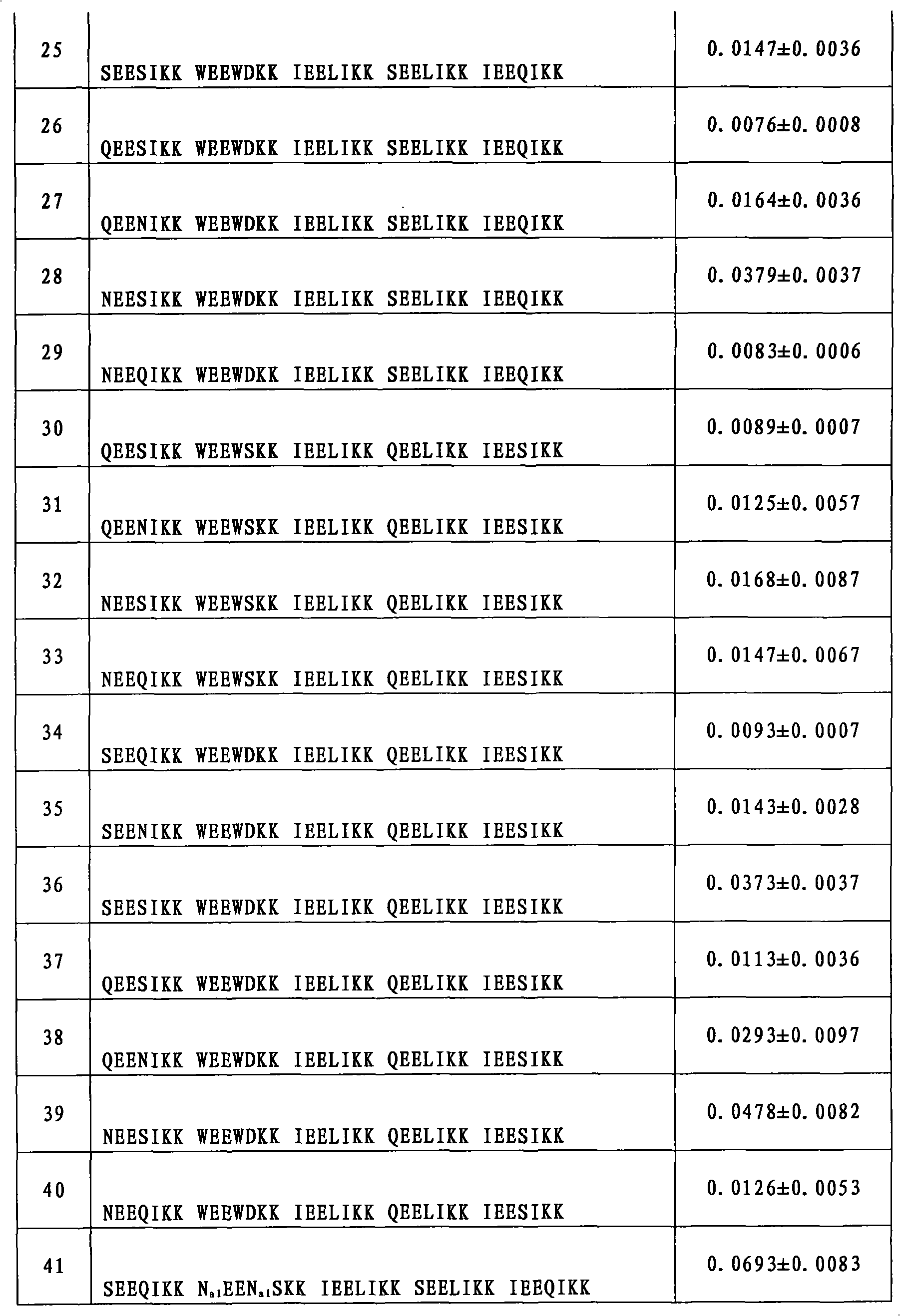
[0157] Example 69: Inhibition of HIV-1 Biological Activity Detection
[0158] Evaluation of compounds inhibiting HIV-1-mediated cell-cell fusion activity (IC 50 ):
[0159] Detection of HIV-1-Mediated Cell-Cell Fusion by Stain Transfer Assay: HIV-1 IIIB Infected H9 cells (H9 / HIV-1 IIIB ) was labeled with a fluorescent reagent Calcein-AM (MolecularProbes, Inc., Eugene, OR), and then, 50 μl of labeled H9 / HIV-1 was added to each well of a 96-well plate IIIB cells (2×10 5 / ml), and then add 50 μl of different concentrations of test samples (compounds prepared in the tested examples 1-34 and 44-53, two-fold serial dilution from a concentration of 250 μg / ml), and incubate at 37°C for 30min; then each well Add 100ul of MT-2 cells (1×10 6 / ml), incubated at 37°C for 2h. Confused and unfused Calcein-labeled HIV-1 infected MT-2 cells (MT-2 is a commonly used effector cell used in this experiment to simulate HIV-infected target cells, commercially available) with an inverted fluore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com