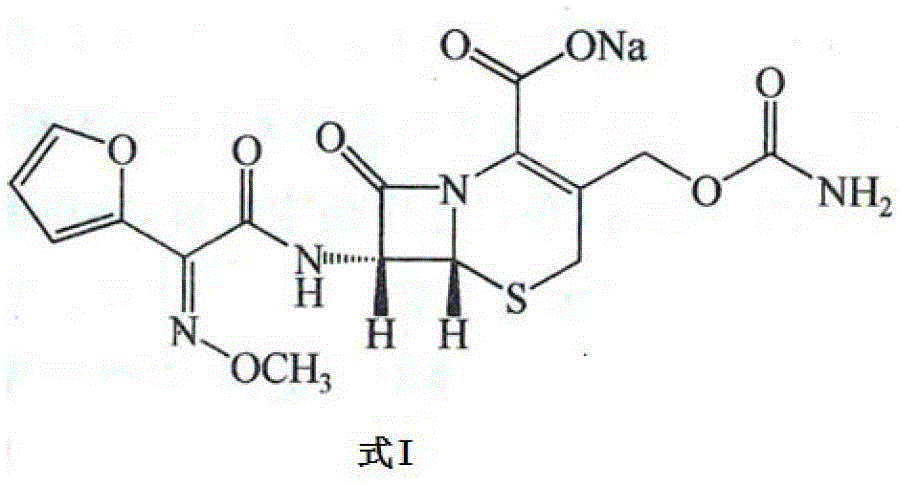
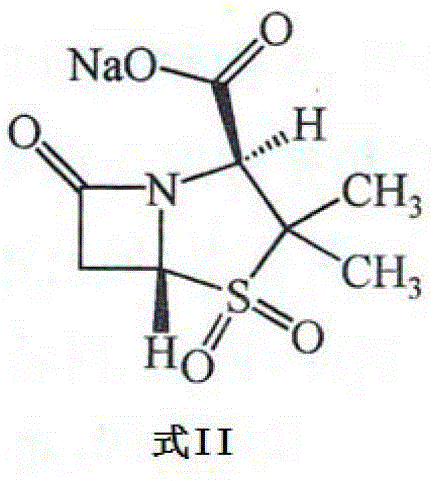
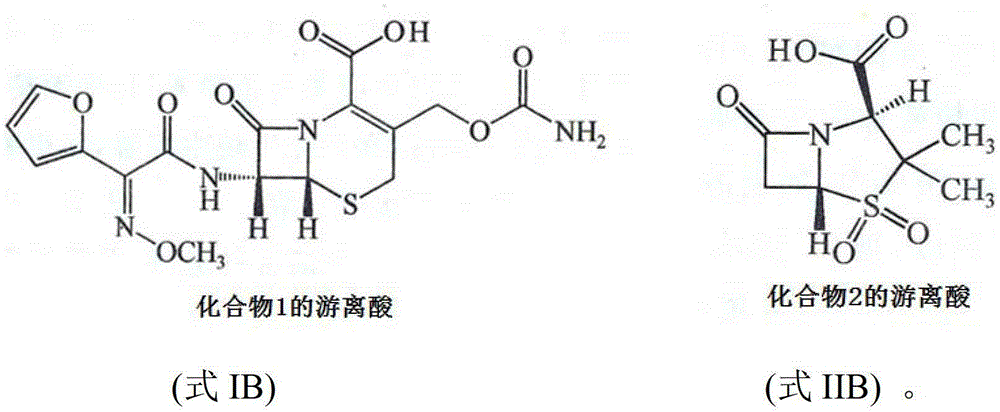
Pharmaceutical composition for improving pharmaceutical stability
A composition and stability technology, applied in the field of medicine, can solve problems such as poor quality stability of pharmaceutical compositions, unsuitable acceptance by patients, and any research on pH of pharmaceutical compositions, etc., to achieve good water solubility and stability, water solubility and stability The effect of excellent performance and good quality stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 The pharmaceutical composition composed of compound 1 and compound 2
[0059] Four batches of compound 1 raw materials and three batches of compound 2 raw materials purchased from different manufacturers were tested according to the standards for compound 1 and compound 2 in the second part of the 2010 edition of "Chinese Pharmacopoeia". Moisture, pH The value, content and related substances all meet the relevant requirements of the standard.
[0060] Get 4 batches of compound 1, each batch respectively gets three groups (the weight of each group is 1500g by the free acid weight of compound 1) and respectively with 3 batches of compound 2 (by the free acid weight of compound 2 weight is 750g) and mixed, using a powder injection aseptic filling machine (a total of 12 batches of pharmaceutical compositions composed of Compound 1 and Compound 2 with a weight of 2250g were obtained), and each batch of Compound 1 and Compound 2 The pharmaceutical composition forme...
Embodiment 2
[0061] Example 2 The pharmaceutical composition composed of compound 1 and compound 2
[0062] With the raw material that embodiment 1 moisture, pH value, content and related substance detection all meet standard relevant requirement, get the compound 1 of 4 batches, each get three groups (each group is by the free acid weight of compound 1) Weight is 1500g), is mixed with 3 batches of compound 2 (the weight by the free acid weight of compound 2 is 500g) respectively, adopts powder injection aseptic dispensing machine (the weight that obtains 12 batches altogether is the compound of 2000g 1 and compound 2) to be subpackaged, each batch of compound 1 and compound 2 is divided into 1000 vials, and the amount of compound 1 in each bottle is calculated as the free acid of compound 1 The weight ratio of compound 2 in terms of the free acid of compound 2 is 3:1, the total weight of the free acid of compound 1 and the free acid of compound 2 is 2.0g, that is, each bottle contains 1.5...
Embodiment 3
[0063] Example 3 The pharmaceutical composition composed of compound 1 and compound 2
[0064] With the raw material that embodiment 1 moisture, pH value, content and related substance detection all meet standard relevant requirement, get the compound 1 of 4 batches, each get three groups (each group is by the free acid weight of compound 1) Weight is 2000g), respectively mixed with 3 batches of compound 2 (the weight by the free acid weight of compound 2 is 500g), adopts powder injection aseptic filling machine (obtaining 12 batches in total is the compound that the weight of 2500g 1 and compound 2) to be subpackaged, each batch of compound 1 and compound 2 is divided into 1000 vials, and the amount of compound 1 in each bottle is calculated as the free acid of compound 1 The weight ratio of compound 2 in terms of the free acid of compound 2 is 4:1, the total weight of the free acid of compound 1 and the free acid of compound 2 is 2.5g, that is, each bottle contains 2.0g of f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com