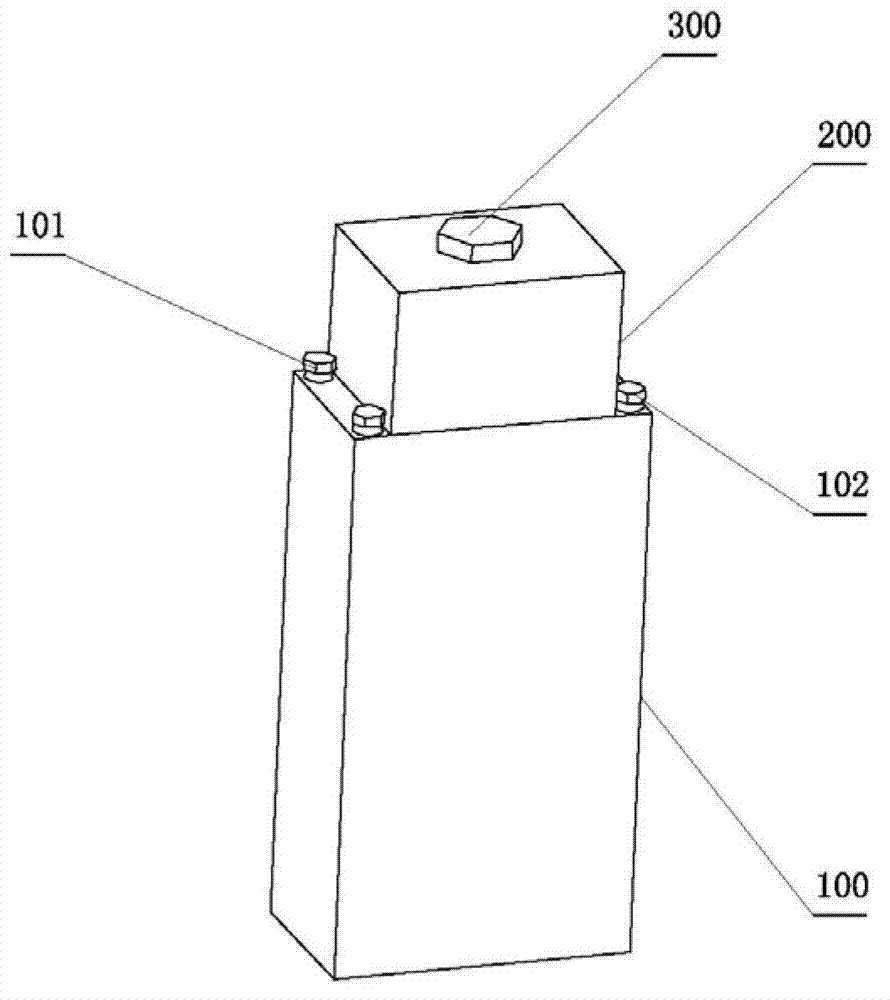
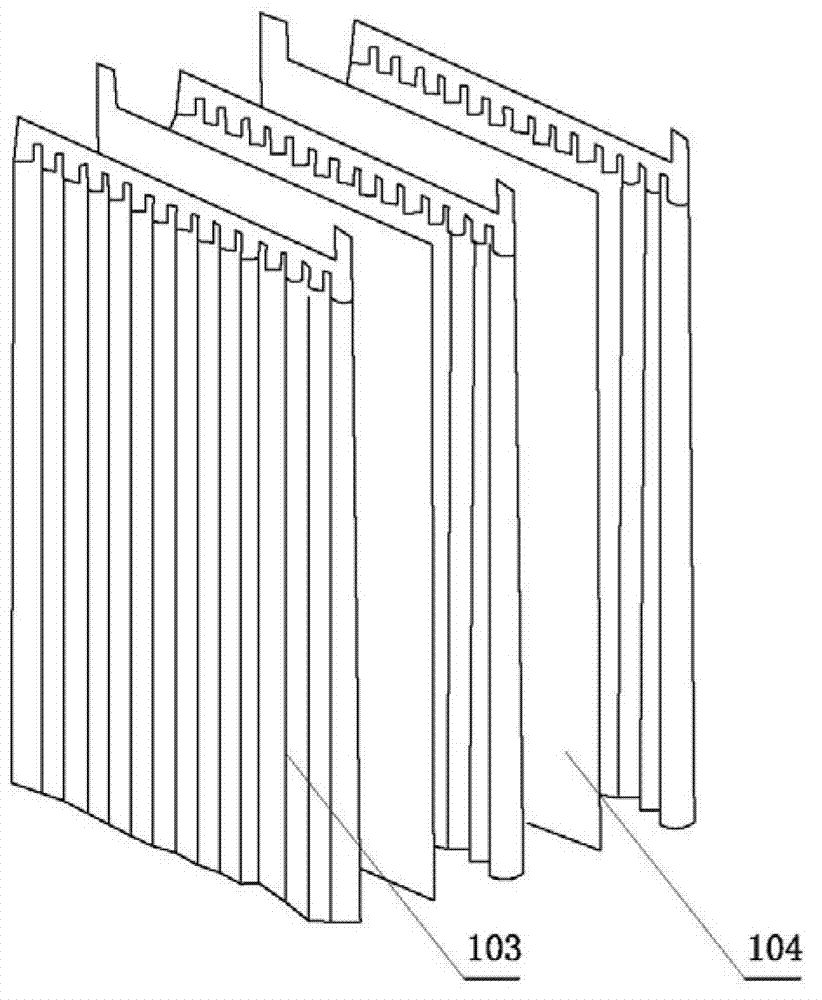
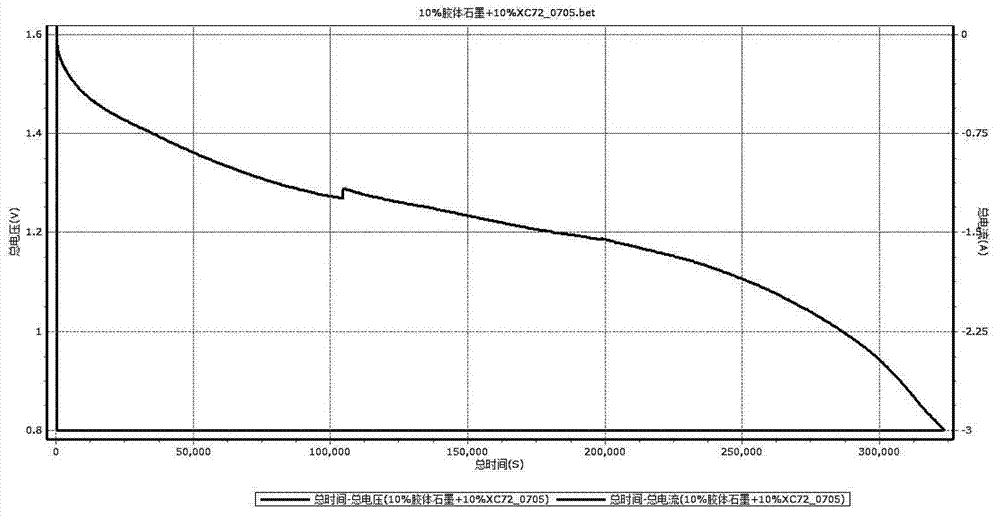
Zinc and manganese reserve battery and manufacture method thereof
A storage battery, zinc-manganese technology, applied in the field of zinc-manganese storage battery and its preparation, can solve the problems of hydrogen evolution, oxygen evolution, unfavorable downhole safety, spontaneous combustion, etc., and achieve the effects of stable performance, wide working temperature range and high output power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The manganese anode comprises electrolytic MnO 2 . Conductive carbon material and additive, the conductive carbon material is colloidal graphite, the additive is SBR, the zinc cathode is zinc sheet, the electrolyte is KOH, and the corrosion inhibitor of the electrolyte is ZnO.
[0053] The preparation method of the zinc-manganese reserve battery described in embodiment 1 comprises the following steps:
[0054] S100: Prepare manganese anode; electrolyze MnO 2, Conductive carbon material and additives are uniformly mixed in a ratio of 80:15:5 by weight, and then the mixture is poured into an alkali-resistant pipe, drawn out through a nickel belt as a current collector and sealed at both ends. Manganese anode; in this step, the homogeneous mixing treatment adopts the electrolytic MnO 2 1. The conductive carbon material and additives are placed in a stirring tank for stirring and dispersing, and the dispersing time is 2 hours.
[0055] S200: preparing a zinc cathode; tai...
Embodiment 2
[0061] The manganese anode comprises electrolytic MnO 2 , conductive carbon material and additive, described conductive carbon material is colloidal graphite, and described additive is SBR, and described zinc cathode is zinc flake, and described electrolytic solution is NaOH, and the corrosion inhibitor of described electrolytic solution is In(OH) 2 .
[0062] The preparation method of the zinc-manganese reserve battery described in embodiment two comprises the steps:
[0063] S100: Prepare manganese anode; electrolyze MnO 2 , colloidal graphite and SBR are uniformly mixed in a ratio of 85:9:6 by weight, then add deionized water to form a paste, and apply the above paste on the current collector; the uniform mixing process is to use ball mill for ball mill dispersion, the dispersion time is 1h; the deionized water and the electrolytic MnO 2 The ratio of copies is 85:20~30;
[0064] S200: Prepare zinc cathode; uniformly mix zinc powder and additives at a ratio of 100:10 to ...
Embodiment 3
[0069] The manganese anode includes electrolytic MnO2 and conductive carbon material, the conductive carbon material is colloidal graphite and carbon nanotubes, the zinc cathode is zinc sheet, the electrolyte is KOH, and the corrosion inhibitor of the electrolyte is ZnO .
[0070] The preparation method of the zinc-manganese reserve battery described in embodiment three comprises the following steps:
[0071] S100: Prepare a manganese anode; uniformly mix electrolytic MnO2, colloidal graphite and carbon nanotubes at a ratio of 75:15:10 by weight, then pour the mixture into an alkali-resistant drain pipe, and pass through nickel as a current collector The manganese anode is made after the tape is drawn and both ends are sealed; the uniform mixing treatment is to use a ball mill for ball milling and dispersing, and the dispersing time is 1h;
[0072] S200: Prepare a zinc cathode; tailor the zinc sheet into a zinc cathode according to the design area, and the design area of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com