Intravaginal devices comprising anticholinergic agents, and methods of making thereof
An internal device and anticholinergic technology, applied in the field of intravaginal devices, can solve the problems of blurred vision, adverse side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Preparation of the first stromal vaginal ring
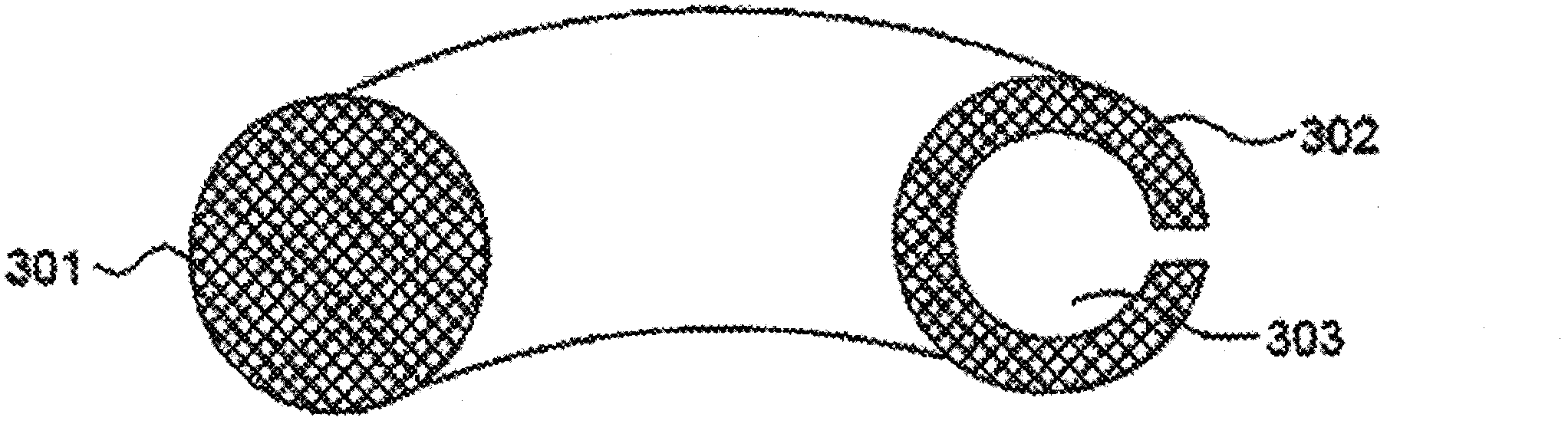
[0096] A vaginal ring comprising a first matrix is prepared as follows. The first matrix was prepared using trifluoropropylmethyl / dimethylsiloxane. In a 100 g capacity Hauschild mixing cup, weigh 40 g of part A and 40 g of part B of trifluoropropylmethyl / dimethylsiloxane elastomeric rubber formation (NuSil Technology, Grade CF2-3521, Toms River, NJ), It was then mixed for 10 seconds in a Hauschild model 501T speed mixer. A metal spatula is then used to scrape down the sides of the jar and further mix the two starting components. Finally, a 14-second accelerated mixing cycle was applied to ensure uniformity of mixing.
[0097]The two halves of the insert mold, which will form the bag and have a bag wall of uniform thickness, are lightly coated in an ethanol / water solution of DARVAN WAQ (R.T. Vanderbilt Co., Norwalk, CT) and air dried. Between 12-15 grams of a 1:1 Part A:Part B mixture was placed into the mold half con...
Embodiment 2
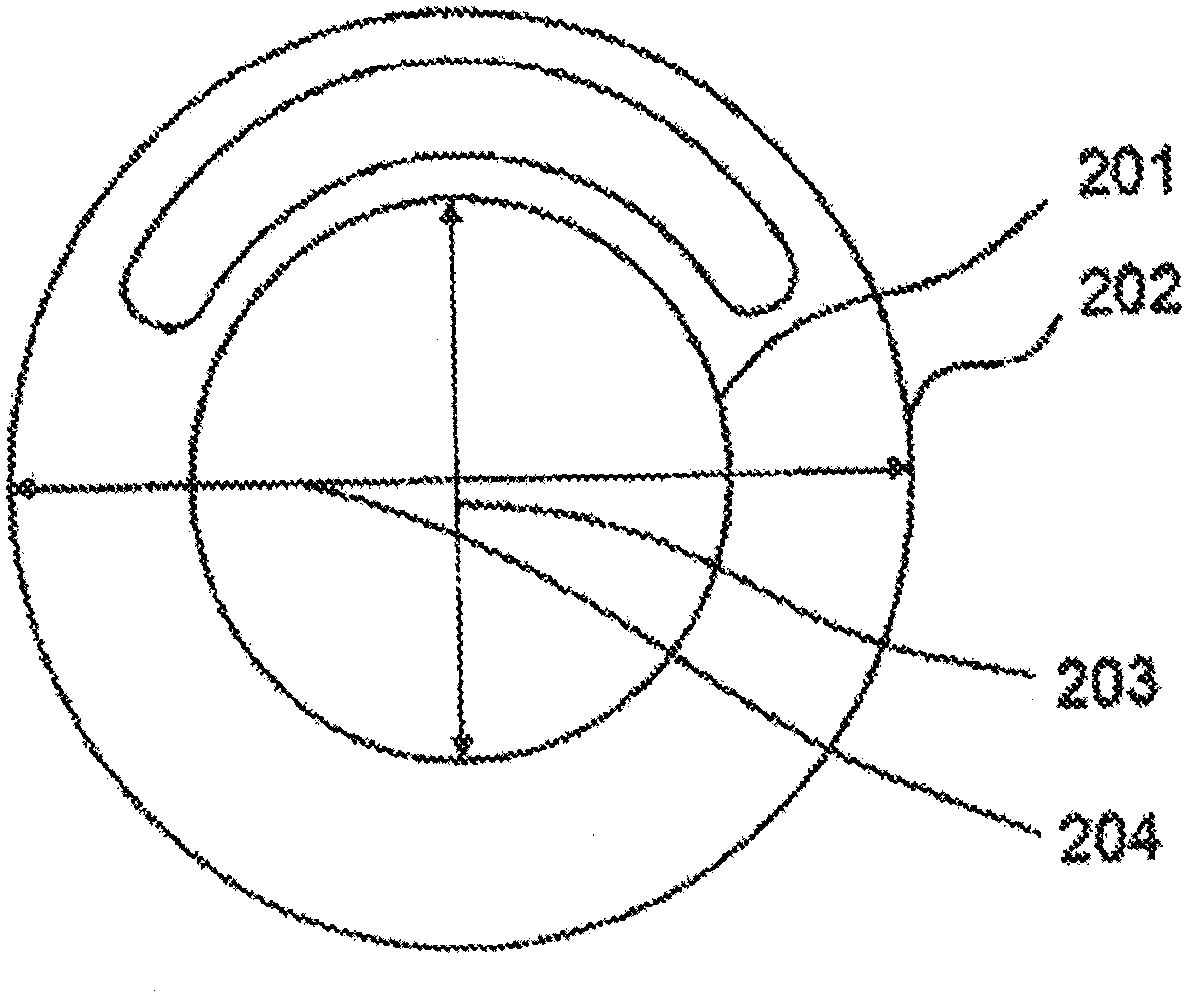
[0103] Preparation of two-matrix vaginal rings
[0104] The pockets of the annular primary matrix of the trifluoropropylmethyl / dimethylsiloxane synthetic rubber prepared according to Example 1 were filled with the silicone / oxybutynin secondary matrix.
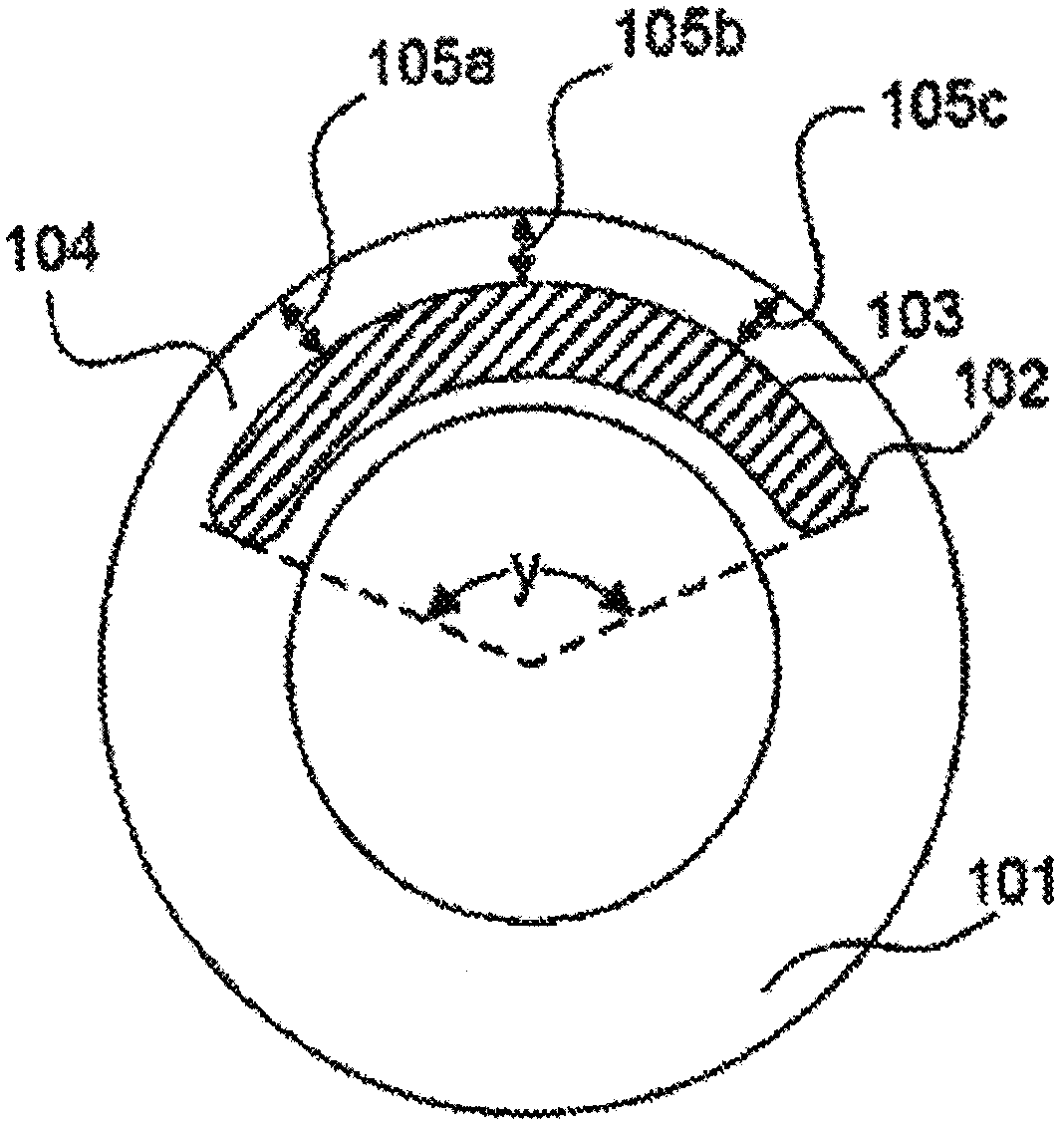
[0105] To form the second matrix, a mixture of 55% silicone and 45% oxybutynin was weighed into a Hauschild mixing cup and mixed in a Hauschild AM 501 Model T speed mixer. The resulting silicone / oxybutynin paste was injected ample amount into the ring pocket of Example 1 using a syringe. In order to obtain a vaginal ring releasing oxybutynin 4 mg / day, a vaginal ring was used containing a first matrix with an outer diameter of 58.3 mm and a pocket extending 80° around the periphery of the ring. The bag is 5.3 mm in diameter and is syringe filled with a silicone / oxybutynin mixture. In order to obtain a vaginal ring releasing 6 mg / day oxybutynin, a vaginal ring was used containing a first matrix with an outer diameter of 58.3 mm...
Embodiment 3
[0108] Pharmacokinetics and Drug Metabolism in Animals
[0109] A study was performed in dogs to determine the levels of oxybutynin and its active metabolite N-desethyloxybutynin in plasma following oral and intravaginal administration of oxybutynin. The results of this study are shown in Table 1.
[0110] Table 1 Oxybutynin vaginal ring and Oxybutynin hydrochloride oral tablet: C max and T max dose comparison of
[0111]
[0112] A 14-day study was conducted in which 8 adult females were randomly assigned to 4 groups of 2 dogs each. Two dogs received 10 mg of oxybutynin hydrochloride orally per day (2 x 5 mg / day tablets) for 14 consecutive days. The remaining 6 dogs received vaginal rings as described in Example 2 designed for sustained release of oxybutynin at doses of 0, 2.5 or 6 mg / day for 14 days.
[0113] Oxybutynin was detected in the plasma of dogs administered orally or intravaginally with oxybutynin at all intervals tested. mean maximum plasma concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com