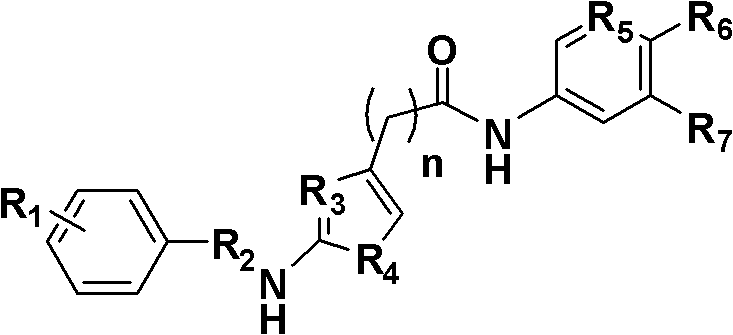
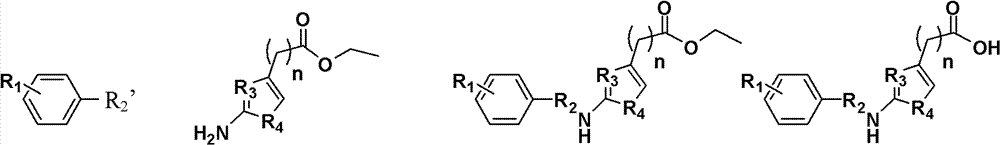
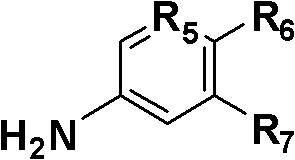
2-aminothiazole-4-amide derivative, its preparation method and application
A reaction and group technology, which is applied in the field of 2-aminothiazole-4-amide derivatives and their preparation, can solve the problems of side effects and drug resistance, and achieve simple reactions, correct structures, and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] Example 1: Preparation of compound N-(2-chloropyridin-5-yl)-2-(2-benzamidothiazol-4-yl)acetamide (R in formula I 1 = H, R 2 =-COCl,R 3 = N, R 4 = S, R 5 = N, R 6 = Cl, R 7 =H, the compound of n=0)
[0037] Step 1): Add 3.9 g (0.02 mol) of ethyl 2-aminothiazole-4-acetate in a 100 ml three-necked flask, add 50 ml of tetrahydrofuran, and add 3.32 ml (0.02 mol) of benzoyl chloride dropwise in an ice bath, Reaction at room temperature for 8 hours, then poured into 100ml of water, with saturated NaHCO 3 Wash with aqueous solution, extract 30ml×3 with dichloromethane, wash with 2M dilute HCl solution, dry over anhydrous sodium sulfate, and concentrate to obtain the product ethyl 2-benzamidothiazole-4-acetate.
[0038]Step 2): put the compound 2-benzoylaminothiazole-4-ethyl acetate obtained in step 1 into a 250ml round bottom flask, add ethanol-water-sodium hydroxide (7:3, v / v; 1.5mol / L) 50ml, refluxed for 0.5 hours, cooled to room temperature, adjusted to pH 4-5, whit...
example 2
[0043] Example 2: Preparation of N-(2-chloropyridin-5-yl)-2-[3-(3,4dichlorophenyl)ureido]thiazole-4-carboxamide (R in formula I 1 =3,4-dichloro, R 2 =-NCO,R 3 =N,R 4 =S, R 5 =N,R 6 =Cl, R 7 =H, compounds with n=0)
[0044] Step 1): add 2-aminothiazole-4-ethyl carboxylate 3.5g (0.02mol) in a 100ml three-necked flask, add 50ml tetrahydrofuran, add 3.7g (0.02mol) of 3,4-dichlorobenzene isocyanate, The reaction was carried out at room temperature for 12 hours, and then poured into 100 ml of water and saturated with NaHCO. 3 Washed with aqueous solution, extracted with dichloromethane 30ml×3, washed with 2M dilute HCl solution, dried over anhydrous sodium sulfate, and concentrated to obtain the product 2-[3-(3,4dichlorophenyl)ureido]thiazole- 4-ethyl formate.
[0045] Step 2): put the compound 2-[3-(3,4dichlorophenyl)ureido]thiazole-4-carboxylic acid ethyl ester obtained in step 1 into a 250ml round bottom flask, add ethanol-water-sodium hydroxide (7:3, v / v; 1.5mol / L) 50ml...
example 3
[0050] Example 3: Preparation of N-(2-hydroxy-1-methoxycarbonylphen-5-yl)-2-[3-(3,4dichlorophenyl)ureido]thiazole-4-carboxamide (Formula I Medium R 1 =3,4-dichloro, R 2 =-NCO,R 3 =N,R 4 =S, R 5 =C,R 6 =OH, R 7 =-COOCH 3 , compounds with n=0)
[0051] Step 1): add 3.9g (0.02mol) of 2-aminothiazole-4-ethyl acetate into a 100ml three-necked flask, add 50ml tetrahydrofuran, add 3.7g (0.02mol) of 3,4 dichlorobenzene isocyanate, room temperature The reaction was continued for 12 hours, then poured into 100 ml of water with saturated NaHCO 3 Washed with aqueous solution, extracted with dichloromethane 30ml×3, washed with 2M dilute HCl solution, dried over anhydrous sodium sulfate, and concentrated to obtain the product 2-[3-(3,4dichlorophenyl)ureido]thiazole- 4-ethyl acetate.
[0052] Step 2): put the compound 2-[3-(3,4dichlorophenyl)ureido]thiazole-4-ethyl acetate obtained in step 1 into a 250ml round bottom flask, add ethanol-water-sodium hydroxide (7:3, 1.5mol / L) 50ml, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com