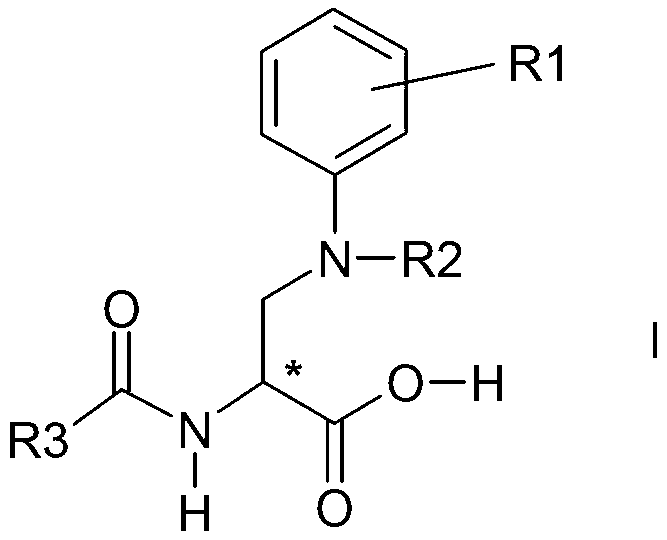
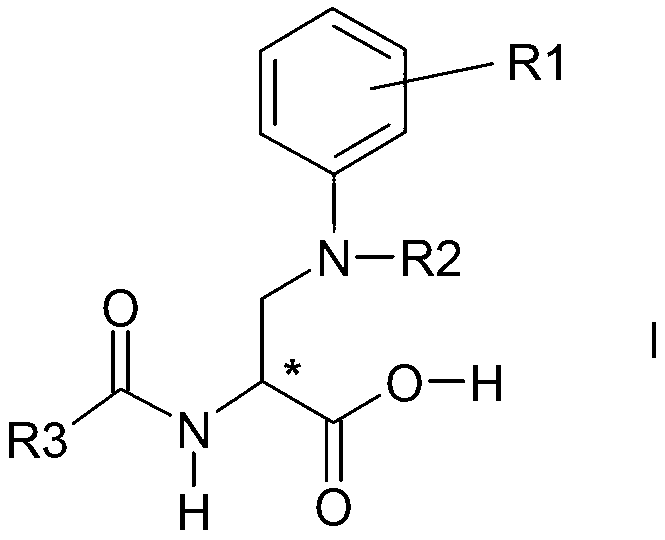
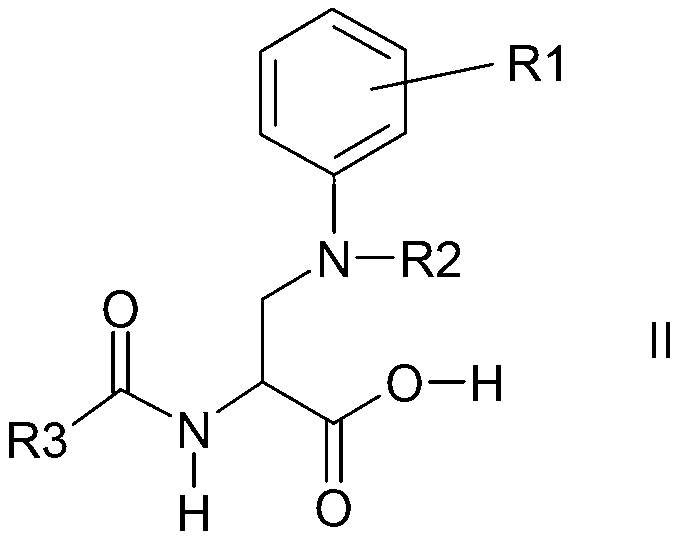
Process for the preparation of enantiomeric forms of 2,3-diaminopropionic acid derivatives
A technology of enantiomers and alkyl groups, applied in medical preparations containing active ingredients, pharmaceutical formulations, organic chemistry, etc., can solve the problems of expensive chiral stationary phases, high equipment and time costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] Preparation of 2-tert-butoxycarbonylamino-3-(phenyl-pyrimidin-2-ylamino)-propionic acid
[0198] step 1
[0199] A solution of 2-anilino-pyrimidine (71 g, 415 mmol; prepared according to T. Matsukawa et al., Yakugaku Zasshi 1951, 71, 933) and lithium tert-butoxide (14 g, 175 mmol) in tetrahydrofuran (170 ml) was cooled to -5°C . At this temperature 2-[bis(tert-butoxycarbonyl)]amino-prop-2-enoic acid methyl ester (100 g, 332 mmol; according to K. El Abdioui et al., Bull. Soc. Chim. Belg. 1997, 106, 425) in tetrahydrofuran (70 ml), the mixture was further stirred at -5°C for 4 hours and then warmed to room temperature (RT).
[0200] step 2
[0201] The reaction mixture obtained in step 1 was mixed with 23% sodium hydroxide solution (290 g, 1.6 mol) and heated under reflux for 7 hours. Tetrahydrofuran was distilled off, and the reaction solution was cooled to room temperature and extracted with methyl tert-butyl ether (400 ml). The aqueous phase was diluted with isopr...
Embodiment 2
[0204] Preparation of (S)-2-tert-butoxycarbonylamino-3-(phenyl-pyrimidin-2-ylamino)-propionic acid (S)-1-phenylethyl-ammonium
[0205] step 1
[0206]2-tert-butoxycarbonylamino-3-(phenyl-pyrimidin-2-ylamino)-propionic acid (120 g, 335 mmol) as prepared in Example 1 was dissolved in n-butyl acetate (1000 ml) at 50° C. The suspension in (S)-phenylethyl-amine (38.5 g, 318 mmol; 99.7% chemical purity, 99.3% ee (enantiomeric excess); BASF) was mixed and the mixture was further stirred at this temperature Cool to room temperature after 2 hours. The precipitated solid was filtered off with suction and dried to give a beige crystalline crude product (83.9 g), which was used directly in Step 2.
[0207] step 2
[0208] The crude product (83.9 g) obtained in step 1 was heated in ethyl acetate (590 ml) under reflux for 2 hours and then cooled to 5°C. The solid was filtered off with suction and dried to give (S)-2-tert-butoxycarbonylamino-3-(phenyl-pyrimidin-2-ylamino)-propionic acid ...
Embodiment 3
[0211] Preparation of (S)-2-tert-butoxycarbonylamino-3-(phenyl-pyrimidin-2-ylamino)-propionic acid
[0212] (S)-2-tert-butoxycarbonylamino-3-(phenyl-pyrimidin-2-ylamino)-propionic acid (S)-1-phenylethyl-ammonium (37.2g, 77.5mmol, 99.5 A suspension of %de) in aqueous isopropanol (50% strength, 90ml) was mixed with 1N sulfuric acid (97ml, 48.5mmol) and stirred at room temperature for 1 hour. The solid was filtered off with suction and dried to give (S)-2-tert-butoxycarbonylamino-3-(phenyl-pyrimidin-2-ylamino)-propionic acid (26.5 g, 95%, >99.9%ee).
[0213] C 18 h 22 N 4 o 4 , M=358.40g / mol; MS (ESI): m / z=359 ((M+1) + , 100%); 1 H-NMR (400MHz, DMSO-d 6 ): δ=12.60(bs, 1H), 8.36(d, J=4.8Hz, 2H), 7.42-7.36(m, 2H), 7.30-7.21(m, 3H), 7.05(t, J=8.3Hz, 1H), 6.76(t, J=4.8Hz, 1H), 4.48-4.30(m, 2H), 4.12-4.01(m, 1H), 1.31(s, 9H)ppm; HPLC (column: Chiralpak AD-H, 250x4.6mm, n-heptane+0.1%TFA: ethanol+0.1%TFA95:5, 1ml / min, 254nm): retention time [min]=19.1(S), 21.4(R).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com