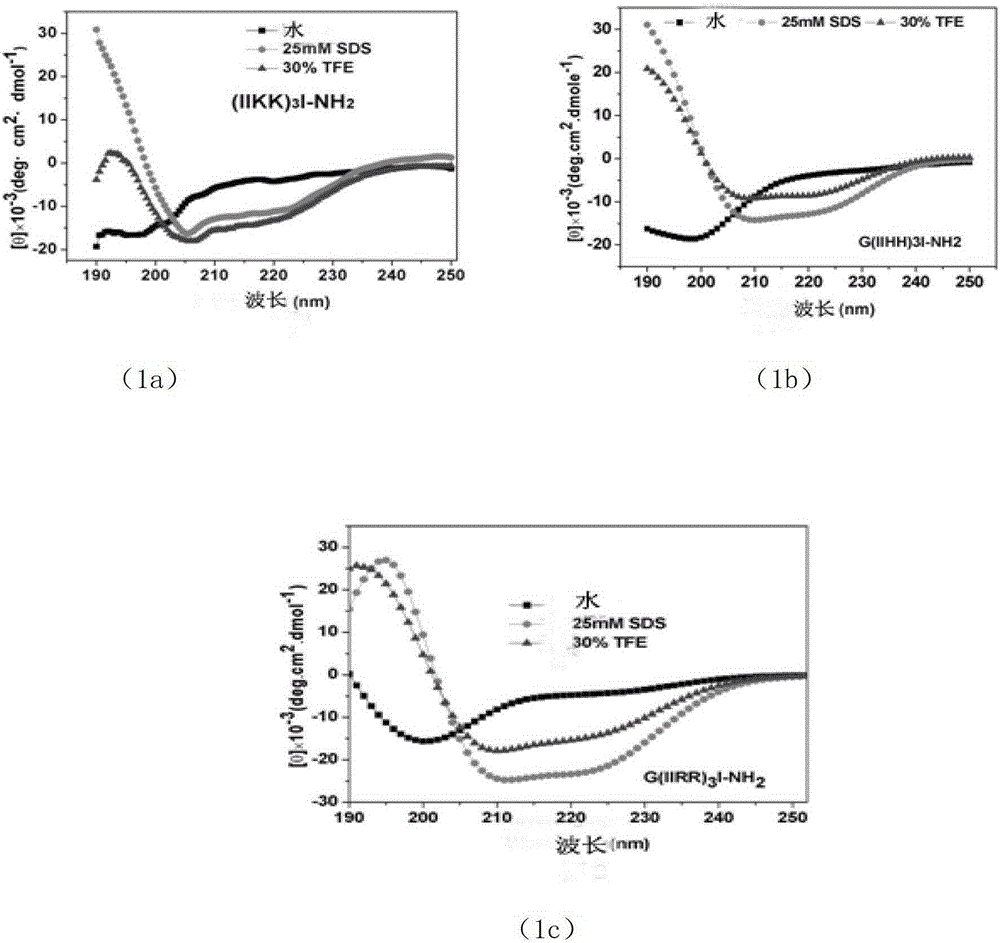
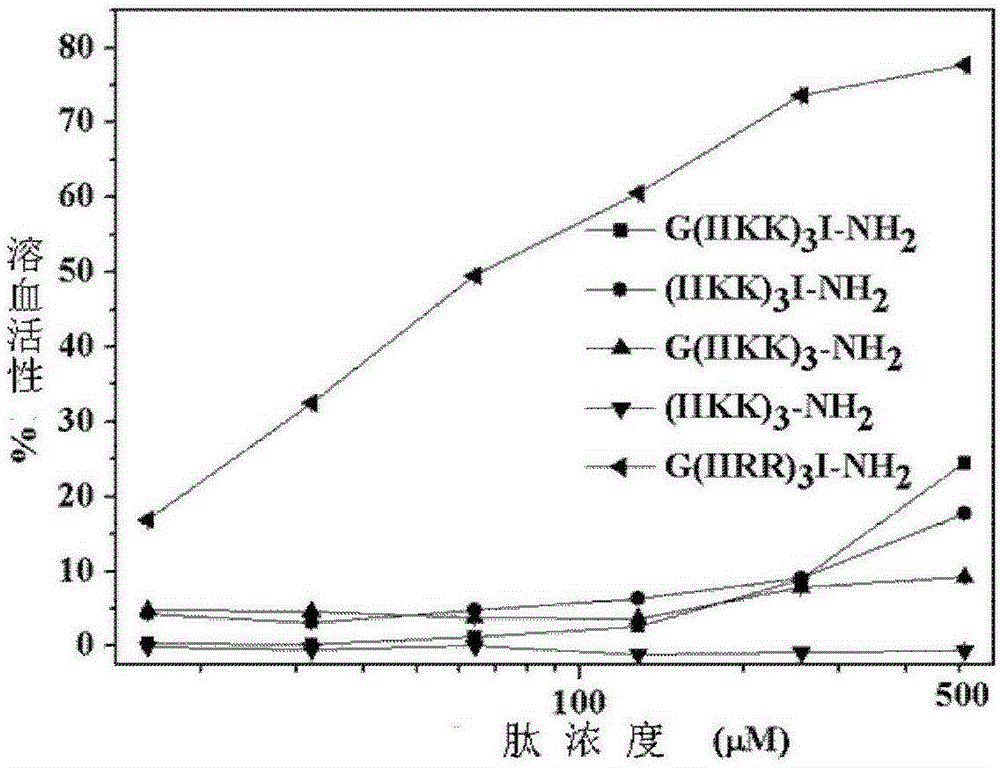
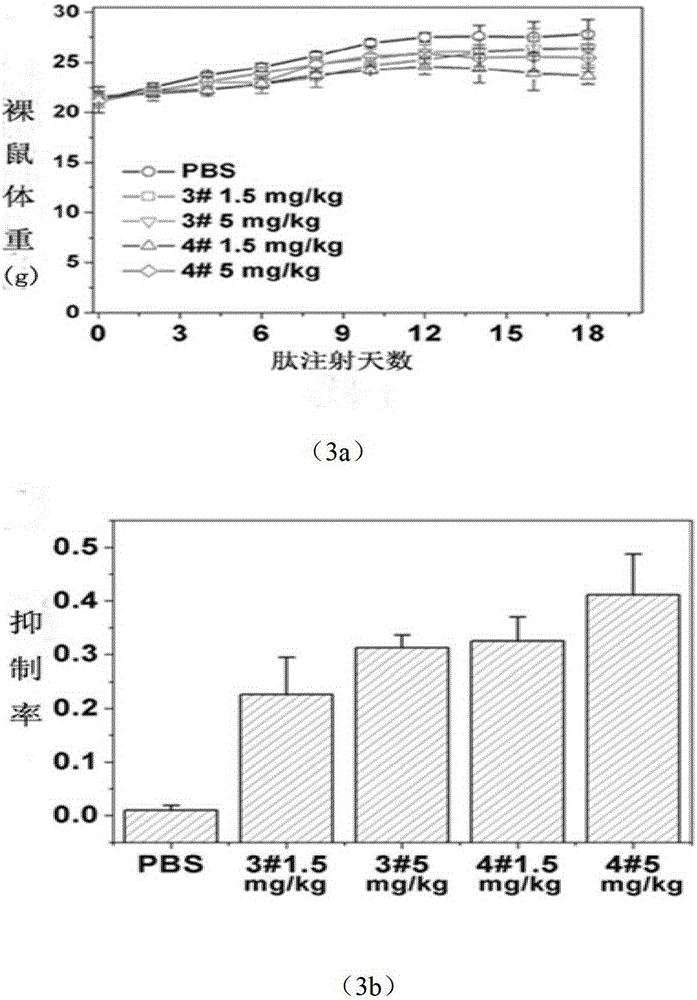
A new type of antimicrobial peptide
An antimicrobial peptide and antibacterial technology, applied in the field of antimicrobial peptides and antibacterial peptides with a helical structure, can solve problems such as complex structure, unfavorable structure-activity relationship, and antibacterial peptide exploration, and achieve high antitumor activity, stable structure, and high antibacterial active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0051] Embodiment 1: the present invention is described in further detail below in conjunction with specific embodiment:
Embodiment 1
[0053] As in formula (b) above, where, A 1 、A 2 、A 3 Both selected as I, B 1 , B 2 All selected as R, n selected as 3, its amino acid sequence is as follows:
[0054] G (IIRR) 3 I;
[0055] The structural formula of its antimicrobial peptide is G(IIRR)3 I-NH 2 ;
[0056] G above (IIRR) 3 I-NH 2 The preparation method:
[0057] Step 1:: Prepare DCM solvent
[0058] Distill industrial DMF (dimethylformamide) under reduced pressure at 60°C, remove about 10mL of the front and rear liquids to obtain pure DMF solvent; perform vacuum distillation on industrial DCM (dichloromethane), first add a small amount of CaH 2 Water-absorbing agent, suspended for 2-3 hours, and then distilled at atmospheric pressure to obtain pure DCM (dichloromethane) solvent.
[0059] Step 2: Weigh the medicine
[0060] Calculated on the antimicrobial peptide synthesizer to prepare 0.25mmolG (IIRR) 3 I-NH 2 The amount of medicines required: (the amount of amino acid is 0.2M, in order to ensure ...
Embodiment 2
[0074] As in formula (c) above, where, A 1 、A 2 Both selected as I, B 1 , B 2 All selected as K, n selected as 3, its amino acid sequence is as follows:
[0075] G(IIKK) 3 ;
[0076] The structural formula of its antimicrobial peptide is G(IIKK) 3 -NH 2 ;
[0077] Above G(IIKK) 3 -NH 2 The preparation method:
[0078] Step 1, step 3 are all the same as specific embodiment 1, and step 2 differs from embodiment 1 step 2 in that:
[0079] Gly: 0.65g dissolved in 11mL DMF;
[0080] Lys: 5.90g dissolved in 63mL DMF;
[0081] Ile: 4.45g dissolved in 63mL DMF;
[0082] Resin: 0.417g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com