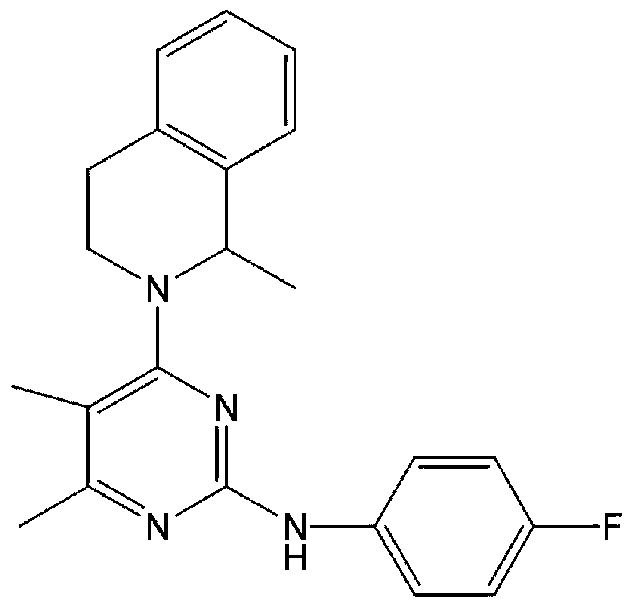
Injectable liquid composition or injectable drypowder containing revaprazan or its salt
A liquid composition and composition technology, applied in the fields of injectable liquid composition and injectable dry powder, can solve the problems of low absorption rate and achieve excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
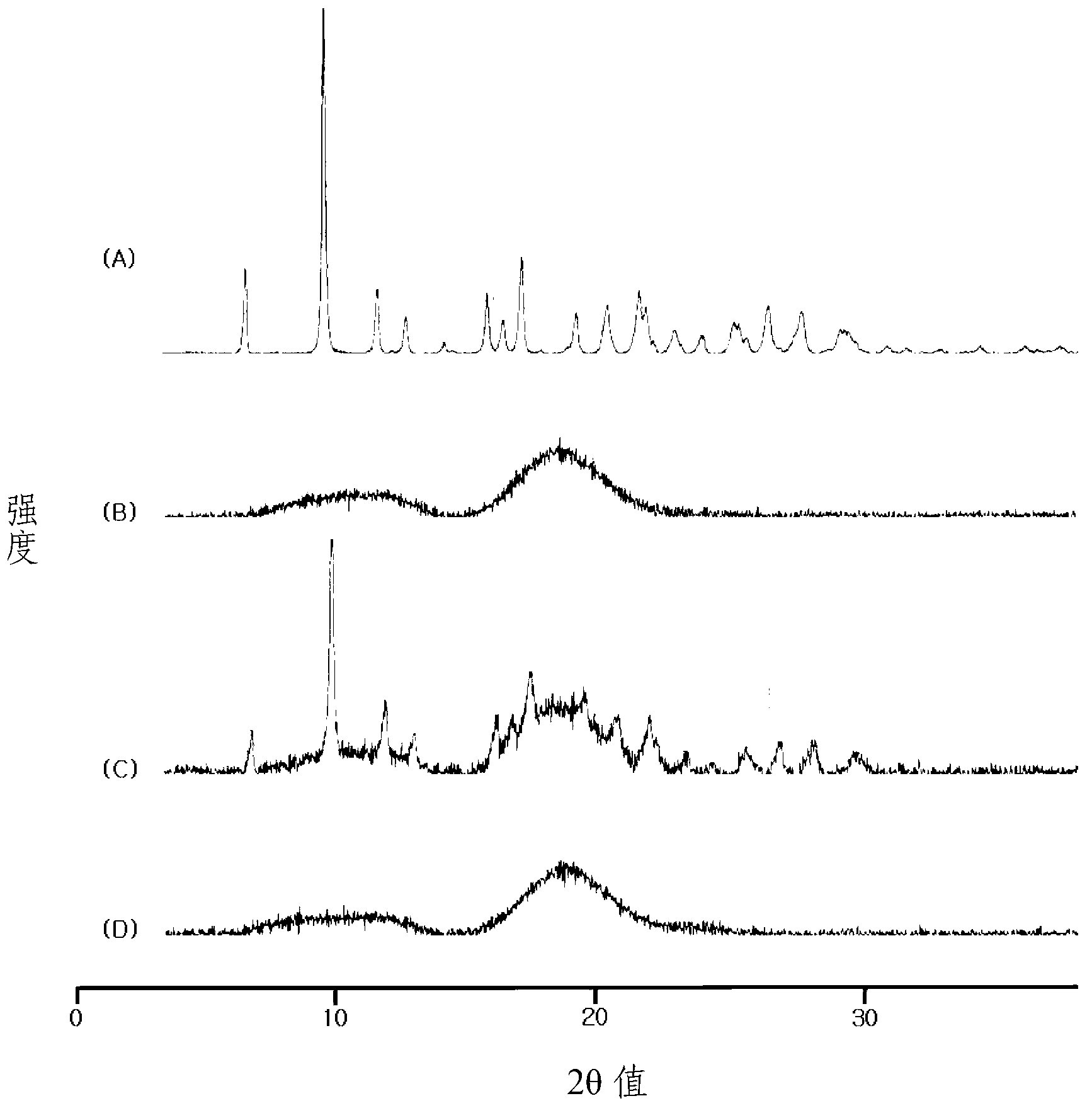
Image
Examples
Embodiment 1-9
[0029] Embodiment 1-9 injection and dry powder for injection
[0030] The inclusion complex of revaprazan hydrochloride and 2-hydroxypropyl-β-cyclodextrin was prepared according to the ingredients and contents shown in Table 1. Revaprazan hydrochloride was added to each ethanol solution of 2-hydroxypropyl-β-cyclodextrin. The resulting mixture was then stirred until complete dissolution. Ethanol was used to obtain a solution with a concentration of 10 w / v% of the obtained components (ie revaprazan + 2-hydroxypropyl-β-cyclodextrin). The resulting solutions were dried at 35° C. under reduced pressure to obtain white powders, respectively.
[0031] Table 1
[0032] Example
[0033] 7
[0034] Water for injection was added to the inclusion complex to obtain a solution, which was subjected to sterile filtration (Pall Co., Acro50 breather filter with Emflon II membrane, pore size: 0.22 μm) to obtain Valrazan hydrochloride (60mg / 3mL) injection. Likewise, the r...
Embodiment 10-18
[0035] Embodiment 10-18 injection and dry powder for injection
[0036]An inclusion complex of revaprazan hydrochloride and 2-hydroxypropyl-β-cyclodextrin was prepared in the same manner as in Examples 1-9. Add water for injection to the inclusion complex, and then add 180 mg of Solutol to it TM HS15 (ie 6v / v%). The resulting solution was subjected to sterile filtration (Pall Corporation, USA, Acro50 breather filter with Emflon II membrane, pore size: 0.22 μm) to obtain an injection containing revaprazan hydrochloride (60 mg / 3 mL). Likewise, the resulting solutions were freeze-dried to obtain dry powders for injection, respectively.
Embodiment 19-24
[0037] Example 19-24 injection and dry powder for injection
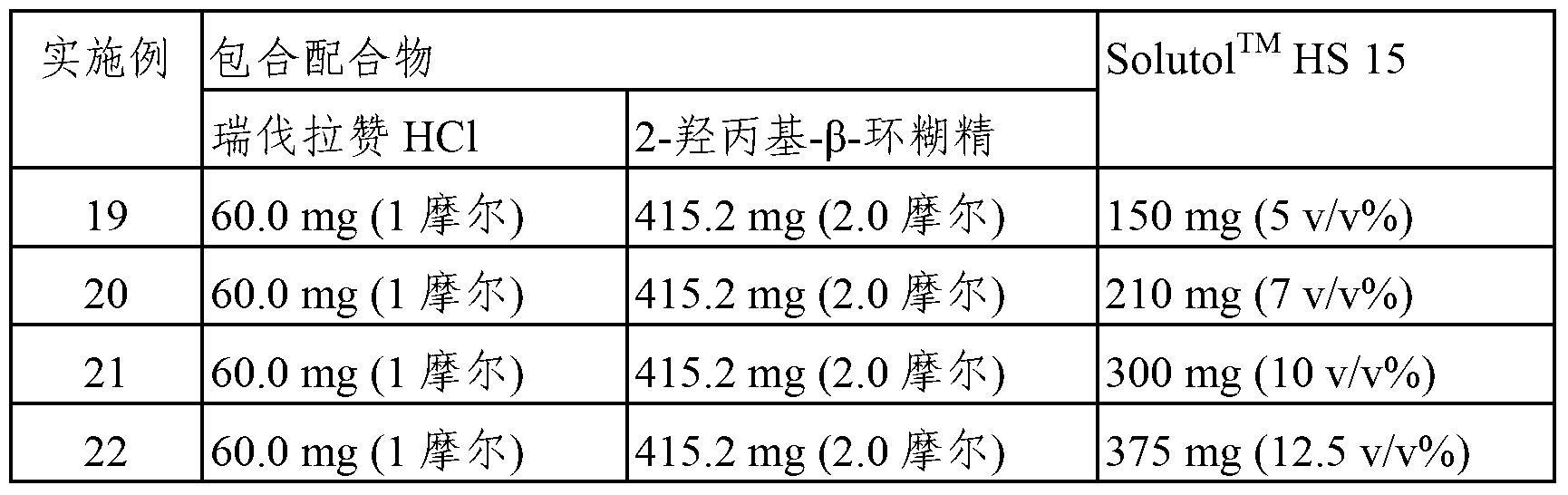
[0038] According to the ingredients and contents shown in Table 2, the inclusion complex of revaprazan hydrochloride and 2-hydroxypropyl-β-cyclodextrin was prepared in the same manner as in Examples 1-9. Water for injection is added to the inclusion complex, and then Solutol is added thereto according to the concentration shown in Table 2 TM HS15. The resulting solution was subjected to sterile filtration (Pall Corporation, USA, Acro50 breather filter with Emflon II membrane, pore size: 0.22 μm) to obtain an injection containing revaprazan hydrochloride (60 mg / 3 mL). Likewise, the resulting solutions were freeze-dried to obtain dry powders for injection, respectively.
[0039] Table 2
[0040]
[0041]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com