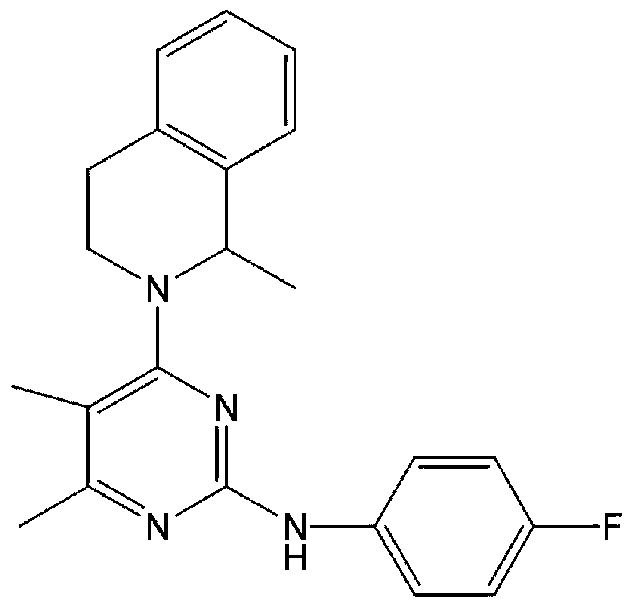
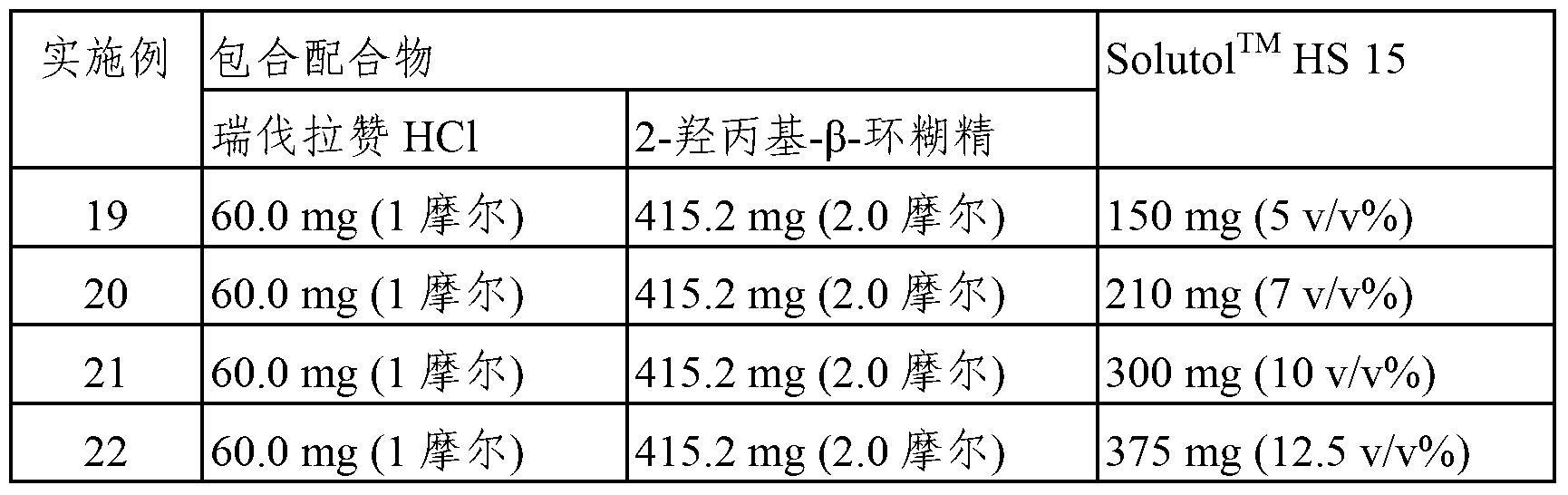
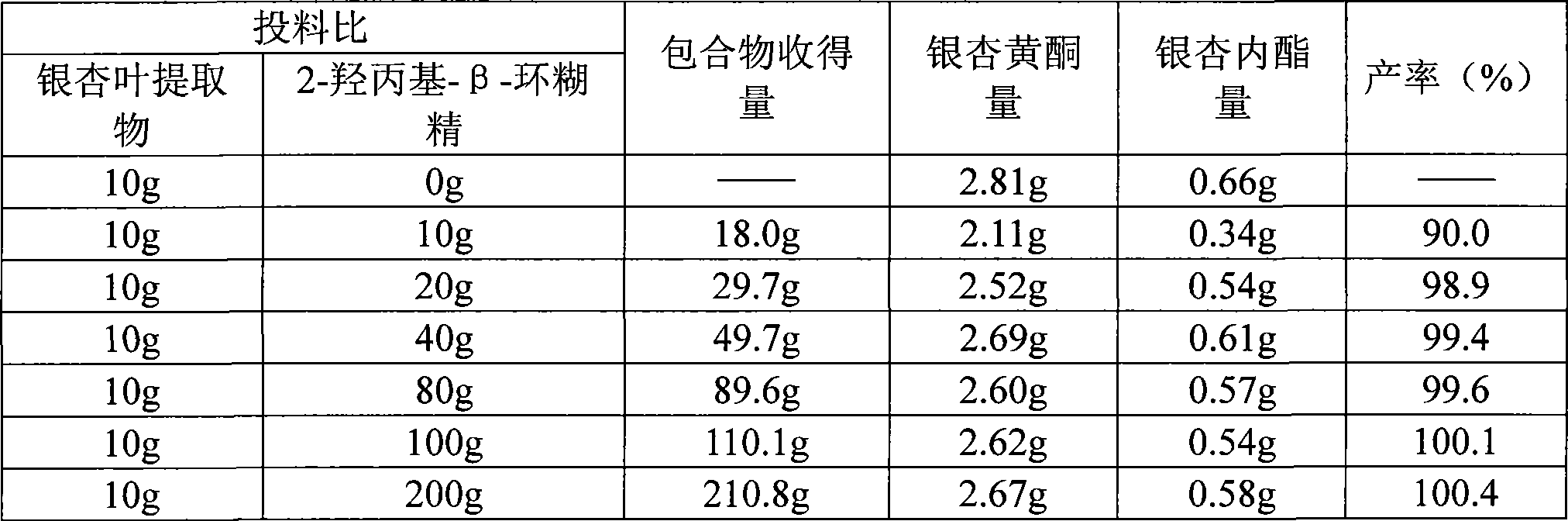
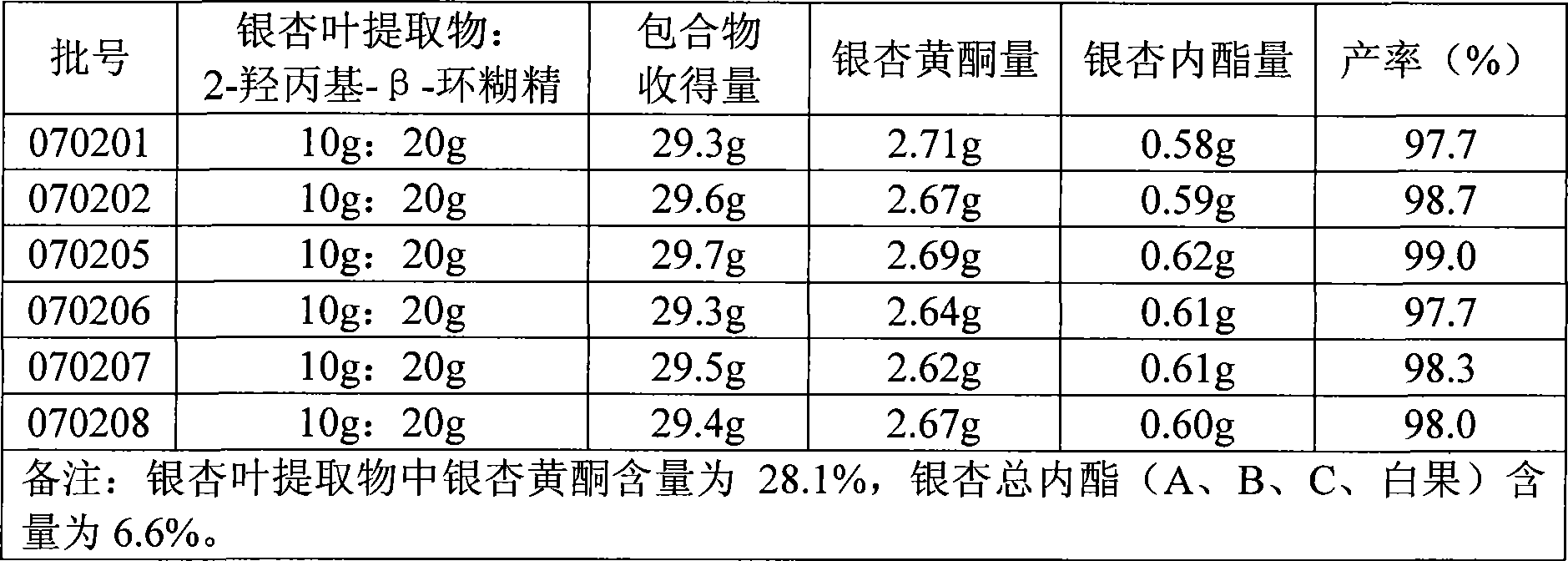
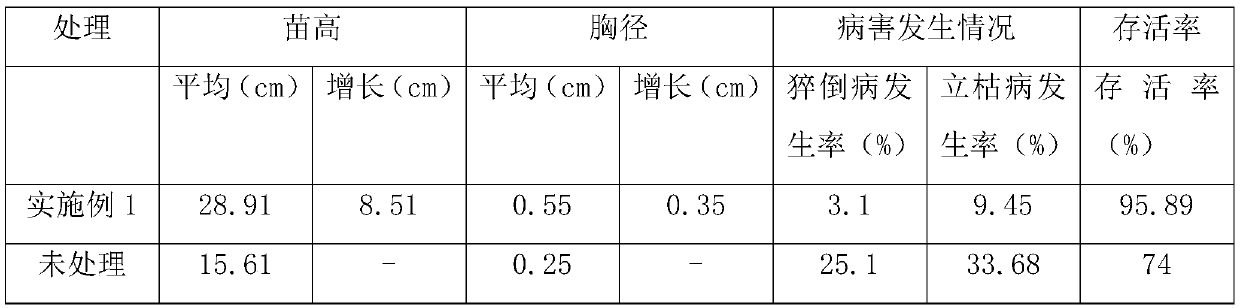
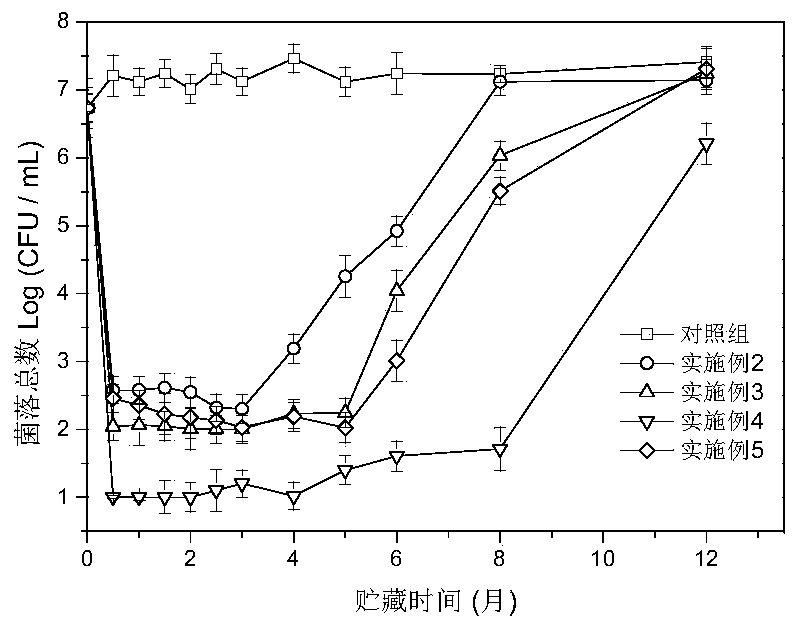
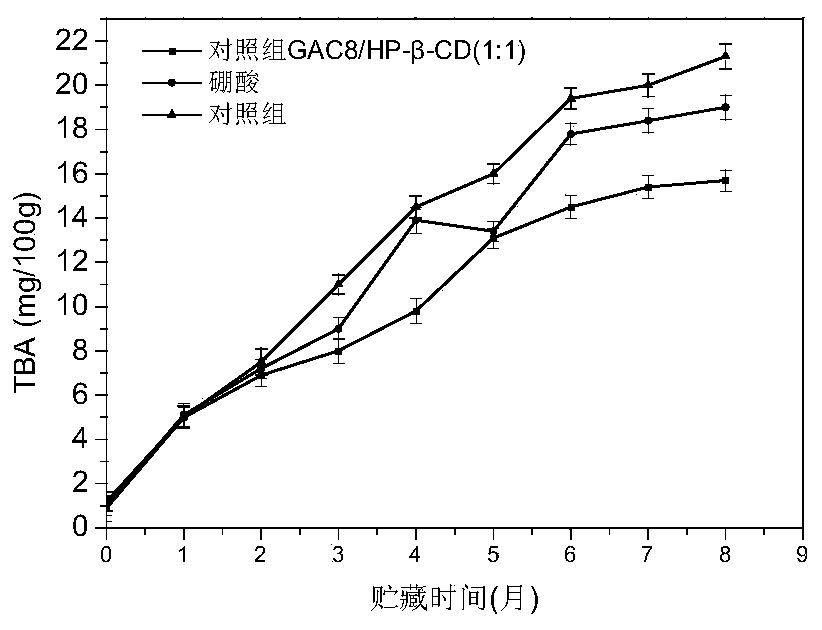
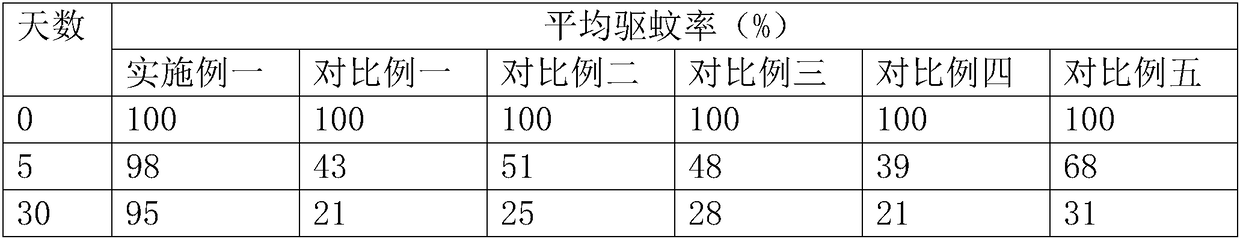
Patents
Literature
46 results about "2-Hydroxypropyl-beta-cyclodextrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Derivative of beta-cyclodextrin that is used as an excipient for steroid drugs and as a lipid chelator.
Method for producing florfenicol soluble powder
InactiveCN101966340AImprove solubilityIncrease productivityAntibacterial agentsOrganic active ingredientsDiseaseSolubility
The invention relates to a method for producing florfenicol soluble powder. The method comprises the following technical steps of: 1, including florfenicol and 2-hydroxypropyl-beta-cyclodextrin; and 2, performing spray drying on included solution by a spray drying tower. By combining grinding inclusion and spray drying process, the production efficiency and the yield are high; the solubility of the prepared florfenicol-included powder is increased to be 263mg / ml (soluble) from original 13mg / ml (slight soluble) and enlarged by 20 times; and the product can be directly dissolved in water and diseases of animals can be treated by drinking the water.
Owner:WUXI ZHENGDA POULTRY
Preparation method of ceftiofur acid long-acting injection
InactiveCN103230364ALong injection half-lifeImprove solubilityAntibacterial agentsOrganic active ingredientsHalf-lifeDissolution
The invention belongs to the technical field of preparation of medicines and in particular relates to a preparation method of ceftiofur acid long-acting injection. The preparation method comprises the followings steps of: adding ceftiofur acid and 2-hydroxypropyl-beta-cyclodextrin in a molar ratio of 1:(1-2) to a ball mill for uniformly mixing; sufficiently grinding under the room temperature, and sufficiently uniformly and sieving to obtain a ceftiofur acid clathrate compound; dissolving sodium alginate in sterile water and adding poloxamer 407 and poloxamer 188, storing for 12-24 hours under the temperature condition of 4 DEG C, so that the poloxamer 407 and the poloxamer 188 are completely dissolved and sterilized under the temperature of 121 DEG C, carrying out ice-bath cooling to obtain a transparent solution; magnetically stirring under the temperature condition of 4 DEG C and the rotation speed condition of 150r / min; adding the ceftiofur acid clathrate compound in a weight ratio of the sodium alginate to the ceftiofur acid clathrate compound of (0.1-0.3):(5-10), so that the ceftiofur acid clathrate compound is sufficiently dispersed uniformly to obtain the ceftiofur acid long-acting injection. According to the preparation method of the ceftiofur acid long-acting injection, the preparation process is simple, the product intramuscular injection half-life period is long, the dissolution degree of the ceftiofur acid is high, the production and treatment cost is low, the cure rate is high and the environment is friendly.
Owner:QINGDAO AGRI UNIV
Pantoprazole sodium medicinal composition and preparation method thereof
ActiveCN102525960AImprove stabilityLow hygroscopicityPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLFreeze-drying
Owner:湖北美林药业有限公司
Preparation method of amphiphilic monodisperse hydroxyapatite monocrystal nanorod
ActiveCN102491300ARaw materials are cheap and easy to getLow costNanotechnologyPhosphorus compoundsApatitePhosphate
The invention relates to a preparation method of an amphiphilic monodisperse hydroxyapatite (HA) monocrystal nanorod. The method comprises: taking the mole ratio 1.67 of calcium element to phosphor element as a reference, taking a soluble calcium salt and a phosphate as the raw materials, adopting a surfactant 2-hydroxypropyl-beta-cyclodextrin (2-HP-beta-CD) for assisting, and employing ethanol and oleic acid as the auxiliary agents, subjecting the materials to reaction in an airtight system for synthesizing a nano-hydroxyapatite particle with good amphiphilic surface properties by controlling the reaction temperature, the reaction time and the raw material ratio, thus obtaining a monodisperse hydroxyapatite monocrystal nanorod with good amphipathy. The nanorod has a diameter of 8-12nm and a length of 150nm-250nm. The hydroxyapatite nanoparticle prepared in the invention can be applied in material outer coatings, degradable artificial bones, bone cement and other biomedical materials, and is an amphiphilic monodisperse hydroxyapatite monocrystal nanorod with excellent biological performance and physico-chemical performance, thus boasting good application prospects.
Owner:SHAANXI GIANT BIOTECHNOLOGY CO LTD
Water-soluble two-photon polymerization initiator as well as assembling method and use thereof
InactiveCN103864964AOvercome remaining deficienciesLow energy for laser processing3-dimensional image productionPhotosensitive materials for photomechanical apparatusLaser processingOrganic solvent
The invention discloses a water-soluble two-photon polymerization initiator as well as assembling method and use thereof, the water-soluble two-photon polymerization initiator is assembled by host molecule 2-hydroxypropyl beta-cyclodextrin and guest molecule 2,7-di(4-pentoxy styrene)-anthraquinone, the molecular formula of the 2,7-di(4-pentoxy styrene)-anthraquinone is described by a formula I as shown in the specification. The water-soluble two-photon polymerization initiator is capable of initiating two-photon polymerization to process three-dimensional hydrogel in a water phase, the laser processing energy is lower in comparison with the processing energy (20-60mW) in the prior art, and the disadvantage of the residue of the organic solvent in the prior art is overcome.
Owner:TIANJIN UNIV
Antibacterial degradable environment-friendly plastic film
InactiveCN107383582AGood environmental effectPromote degradationLinear low-density polyethyleneCarbamate
The invention discloses an antibacterial degradable environment-friendly plastic film. The plastic film is prepared through the following raw materials in parts by weight: 50-60 parts of linear low-density polyethylene, 20-25 parts of polypropylene, 10-15 parts of disulfide carbamate, 10-15 parts of ethyecellulose, 10-15 parts of bamboo carbon powder, 3-7 parts of plasticizer, 5-9 parts of sodium alginate, 10-14 parts of zein, 5-10 parts of tartrate, 3-5 parts of 2-hydroxypropyl-beta-cyclodextrin, 3-5 parts of ASA high-glue powder, 2-4 parts of light degradation agent, 1-3 parts of light stabilizer, and 2-4 parts of cedarwood oil. The plastic film is tough, durable, recyclable, sanitary, harmless, environment-friendly, antibacterial, resistant to moisture and dust, easy to degrade, and outstanding in environmental protection effect; pollution to the land is reduced; the bamboo carbon powder is added in the formula and can release a large number of negative ions, and moreover, the smell absorbing performance, the antibacterial performance and the deodorizing performance are high.
Owner:安徽省天乐塑业有限公司
Frozen dry powder injection of houttuynia cordata and its preparation
A freeze-dried powder injection is prepared from houttuynia through extracting its volatile oil and mixing it with 2-hydroxypropyl-beta-cyclodextrin.
Owner:张晴龙
Injectable liquid composition or injectable drypowder containing revaprazan or its salt
ActiveCN103228279AImprove stabilityOrganic active ingredientsNanomedicineSulfobutylether Beta-CyclodextrinAqueous medium
The present invention provides a liquid composition for injection comprising an inclusion complex of revaprazan or its salt and sulfobutylether-beta-cyclodextrin or 2-hydroxypropyl-beta-cyclodextrin in an aqueous medium; and a dry powder for injection obtained by drying the liquid composition for injection.
Owner:YUHAN
Method for preparing ginkgo leaf extract cyclodextrin inclusion compound and preparation
ActiveCN101502539AImprove the problem of small solubilityImprove stabilityMetabolism disorderPharmaceutical non-active ingredientsSolubilityGinkgo leaf extract
The invention relates to a preparation prepared by ginkgo biloba extract, in particular to a ginkgo biloba extract cyclodextrin inclusion compound and a preparation method of the preparation. The problems of poor solubility, low dissolution rate, poor storage performance and the like in a variety of preparations of the ginkgo biloba extract in the prior art can be solved. The method comprises the steps of adding water in 2-hydroxypropyl-beta-cyclodextrin, heating and dissolving to prepare 2-hydroxypropyl-beta-cyclodextrin solution, then using 95 percent ethanol for dissolving the ginkgo biloba extract, dripping ginkgo biloba extract-ethanol solution to the 2-hydroxypropyl-beta-cyclodextrin solution under the stirring state, lasting the process for 1 hour, stopping heating after completing the dripping, continuously stirring for 1-3 hours; stopping stirring, standing reaction liquid and filtering for preparation. The ginkgo biloba extract 2-hydroxypropyl-beta-cyclodextrin inclusion compound and a variety of preparations can greatly improve the problem of small solubility of the ginkgo biloba extract in the water and improve the stability of the ginkgo biloba extract in the water.
Owner:山西振东泰盛制药有限公司
Preparation method of ceftiofur acid long-acting injection
InactiveCN103230364BLong injection half-lifeImprove solubilityAntibacterial agentsOrganic active ingredientsHalf-lifeDissolution
The invention belongs to the technical field of preparation of medicines and in particular relates to a preparation method of ceftiofur acid long-acting injection. The preparation method comprises the followings steps of: adding ceftiofur acid and 2-hydroxypropyl-beta-cyclodextrin in a molar ratio of 1:(1-2) to a ball mill for uniformly mixing; sufficiently grinding under the room temperature, and sufficiently uniformly and sieving to obtain a ceftiofur acid clathrate compound; dissolving sodium alginate in sterile water and adding poloxamer 407 and poloxamer 188, storing for 12-24 hours under the temperature condition of 4 DEG C, so that the poloxamer 407 and the poloxamer 188 are completely dissolved and sterilized under the temperature of 121 DEG C, carrying out ice-bath cooling to obtain a transparent solution; magnetically stirring under the temperature condition of 4 DEG C and the rotation speed condition of 150r / min; adding the ceftiofur acid clathrate compound in a weight ratio of the sodium alginate to the ceftiofur acid clathrate compound of (0.1-0.3):(5-10), so that the ceftiofur acid clathrate compound is sufficiently dispersed uniformly to obtain the ceftiofur acid long-acting injection. According to the preparation method of the ceftiofur acid long-acting injection, the preparation process is simple, the product intramuscular injection half-life period is long, the dissolution degree of the ceftiofur acid is high, the production and treatment cost is low, the cure rate is high and the environment is friendly.
Owner:QINGDAO AGRI UNIV
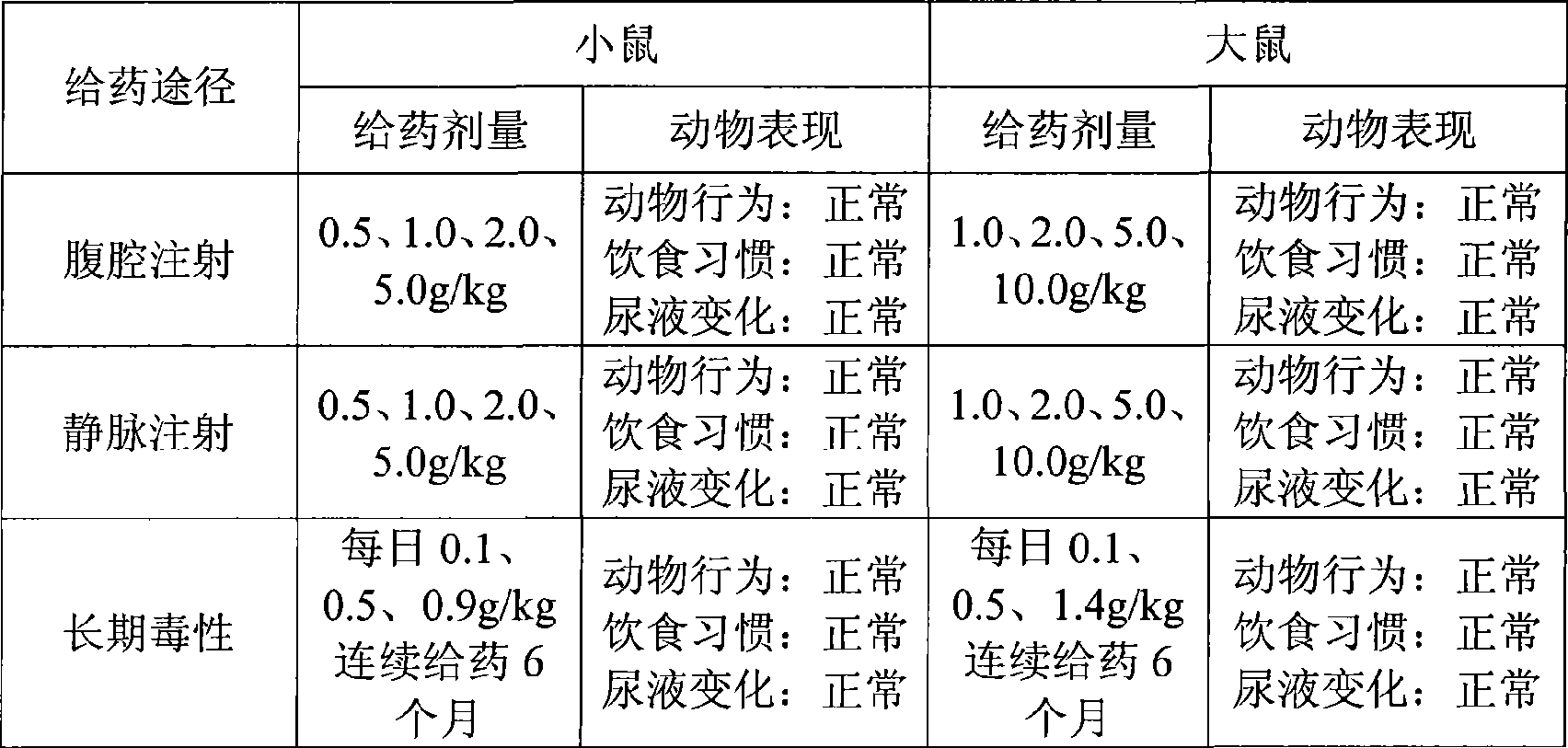
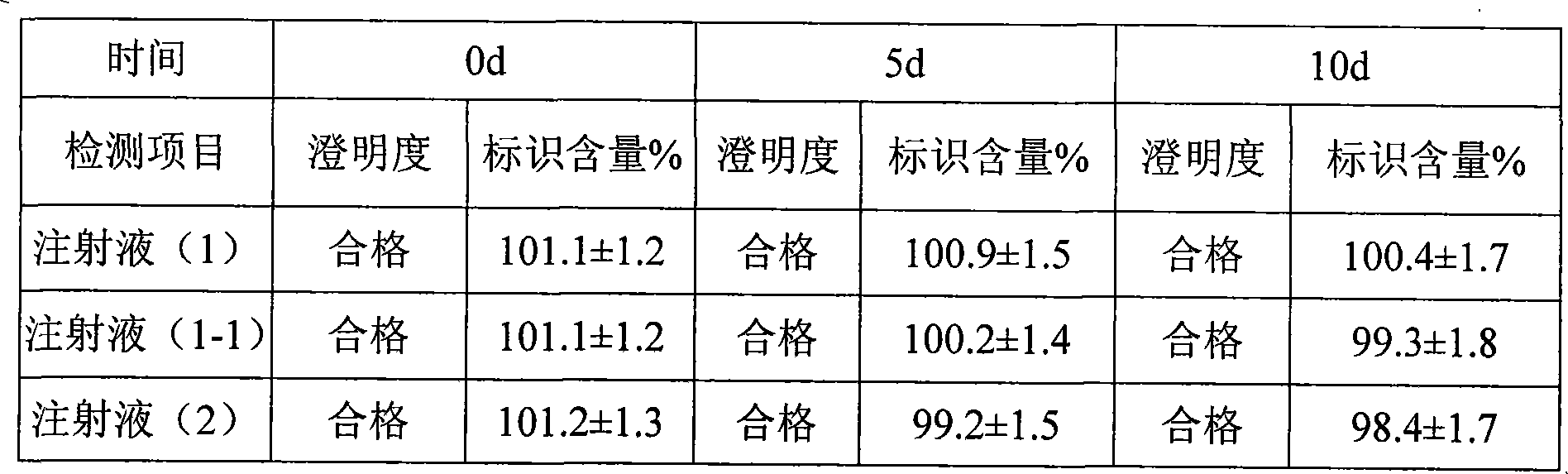
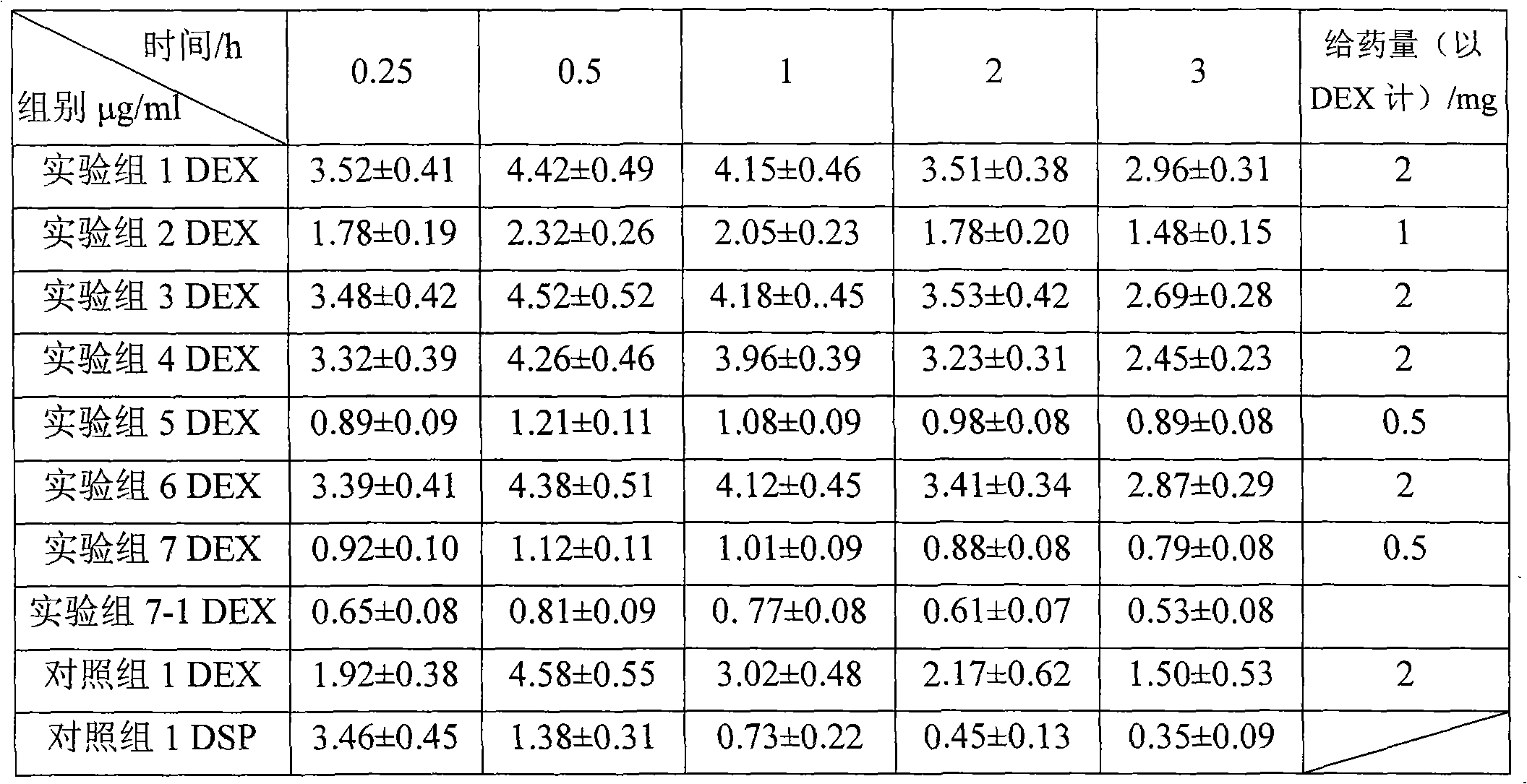
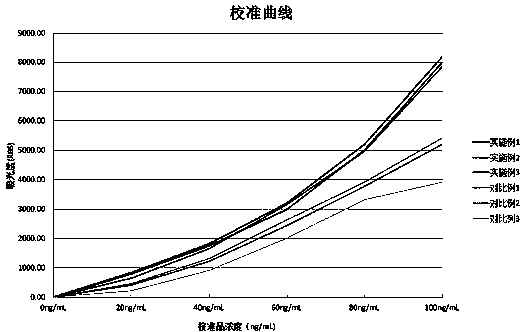
Dexamethasone injecta coated with 2-hydroxypropyl-beta-cyclodextrin
InactiveCN101926760AStable dissociation rateSpeed up dissociationOrganic active ingredientsSenses disorderDexamethasoneCyclodextrin
The invention relates to a dexamethasone injecta coated with 2-hydroxypropyl-beta-cyclodextrin. The molar ratio of a dexamethasone to the 2-hydroxypropyl-beta-cyclodextrin is 1:1-20, the substitution degree of the utilized 2-hydroxypropyl-beta-cyclodextrin is preferably 4-7, and the molar ratio of the dexamethasone to the 2-hydroxypropyl-beta-cyclodextrin is preferably 1:2-5.
Owner:TIANJIN JINYAO GRP
Detection kit for procalcitonin (PCT)
The invention relates to the technical field of procalcitonin (PCT) detection, in particular to a detection kit for procalcitonin (PCT) and a preparation and application method thereof. A reagent R1 contains a buffer solution, potassium chloride, ethyl carbamate, 2-hydroxypropyl-Beta-cyclodextrin, sorbitol, PEG-20000, N, N-diethyl-p-octadecylsulfonimidopropylammonium propanesulfonate, and a preservative. A reagent R2 comprise a buffer solution, ethyl carbamate, 2-hydroxypropyl-Beta-cyclodextrin, sorbitol, rabbit anti-human calcitonin (PCT) antibody-coated latex particles (65nm), rabbit anti-human calcitonin (PCT) antibody-coated latex particles (145nm), rabbit anti-human calcitonin (PCT) antibody-coated latex particles (280nm), N, N-diethyl-p-octadecylsulfopropylammonium propanesulfonate, and a preservative. According to the detection kit for procalcitonin (PCT), the stability and the linear range of the reagent are remarkably improved and the sensitivity and accuracy of the reagentare remarkably enhanced.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Formula for improving water-solubility of armillarisin and its preparation
InactiveCN1415292AGood water solubilityImprove stabilityOrganic active ingredientsDigestive systemSolubilityWater soluble
A process for increasing the water solubility of leucocodin A features that the leucocidin A is mixed with beta-cyclodextrin or 2-hydroxypropyl beta-cyclo dextrin in the ratio of 1:(1-10) to obtain their inclusion compound "leucocidin A-beta cyclodextrin" or "leucocodin A-2 hydroxypropyl beta-cyclodextrin". Its advantages are high safety and high stability.
Owner:SHENYANG PHARMA UNIVERSITY
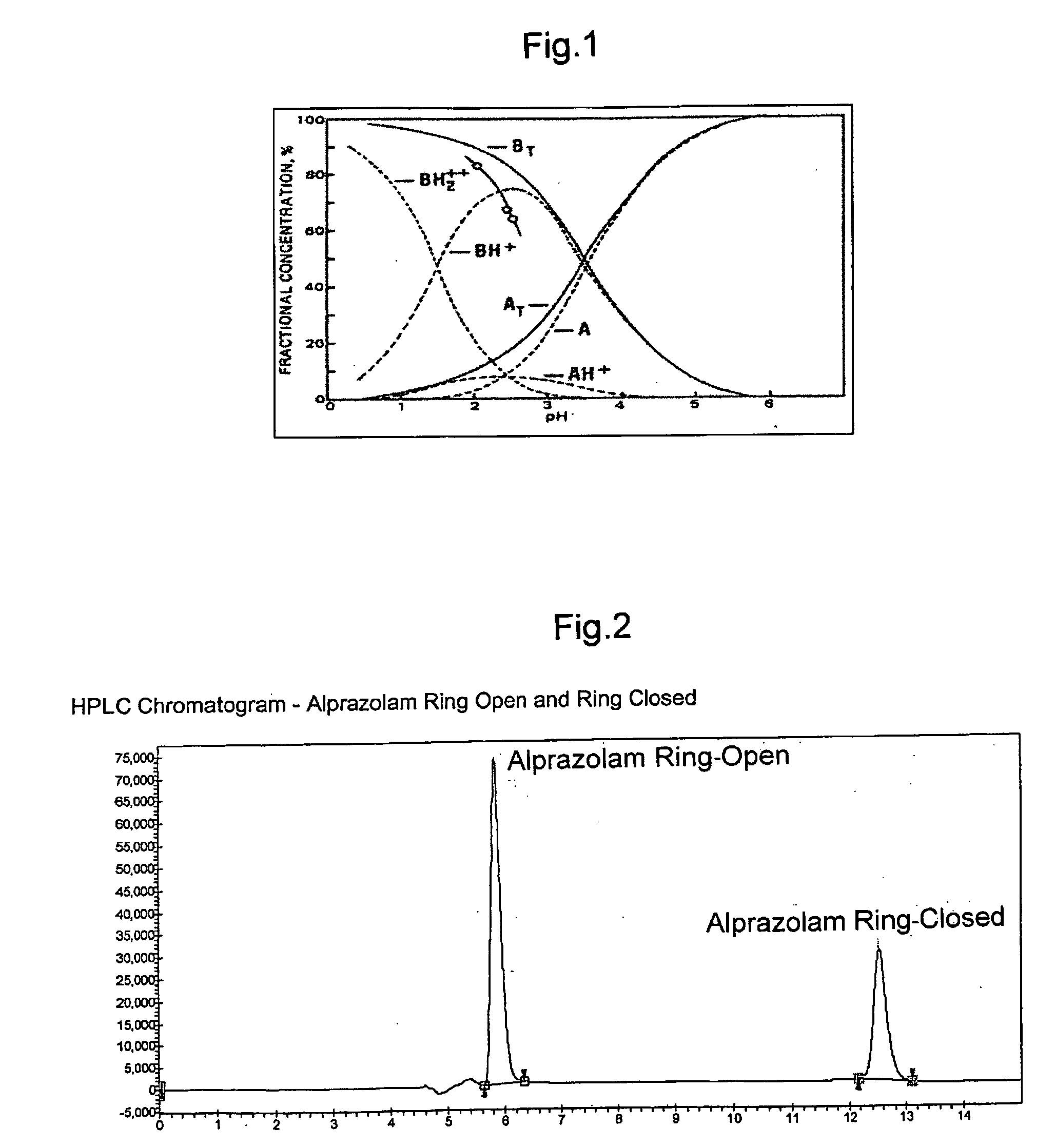
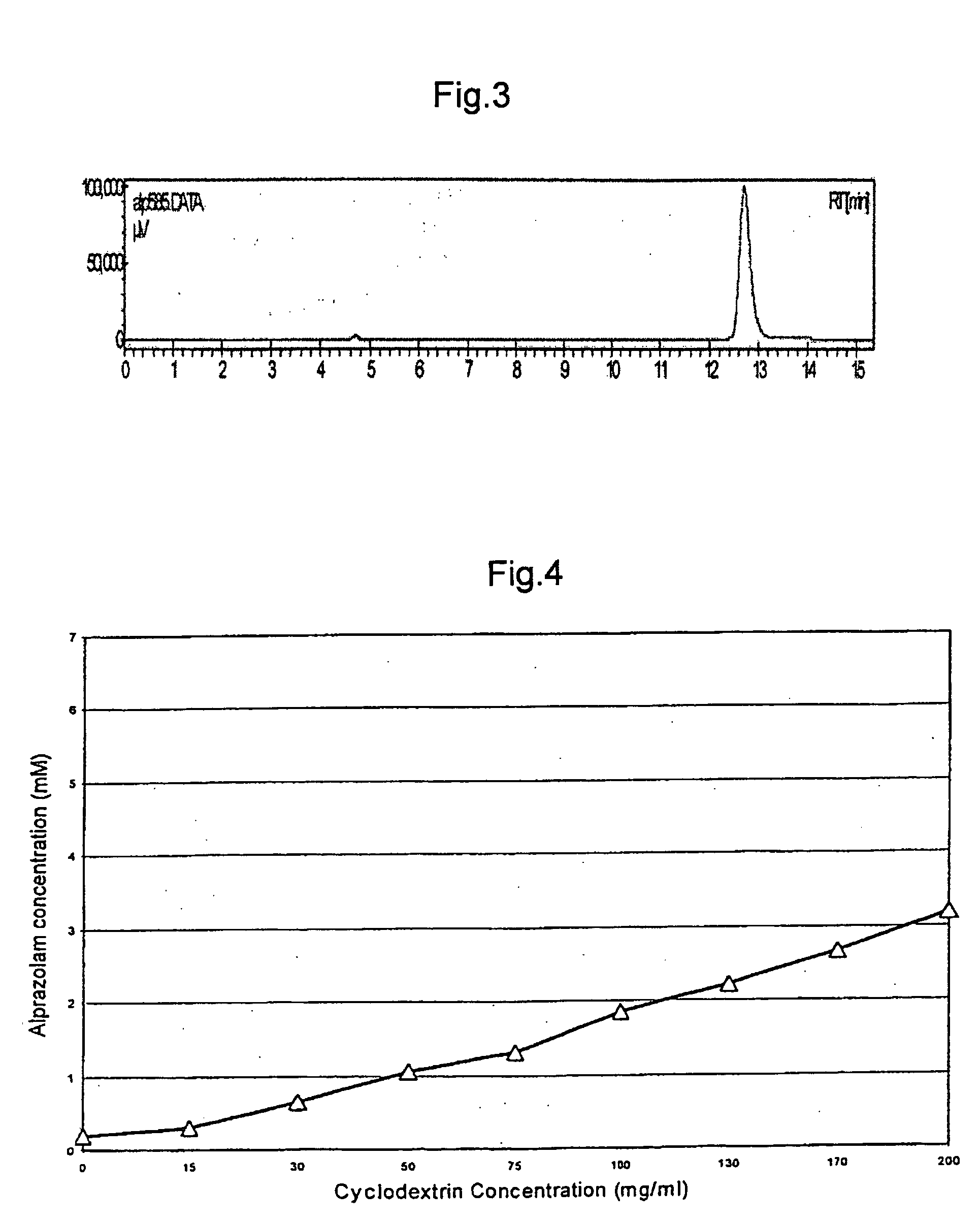
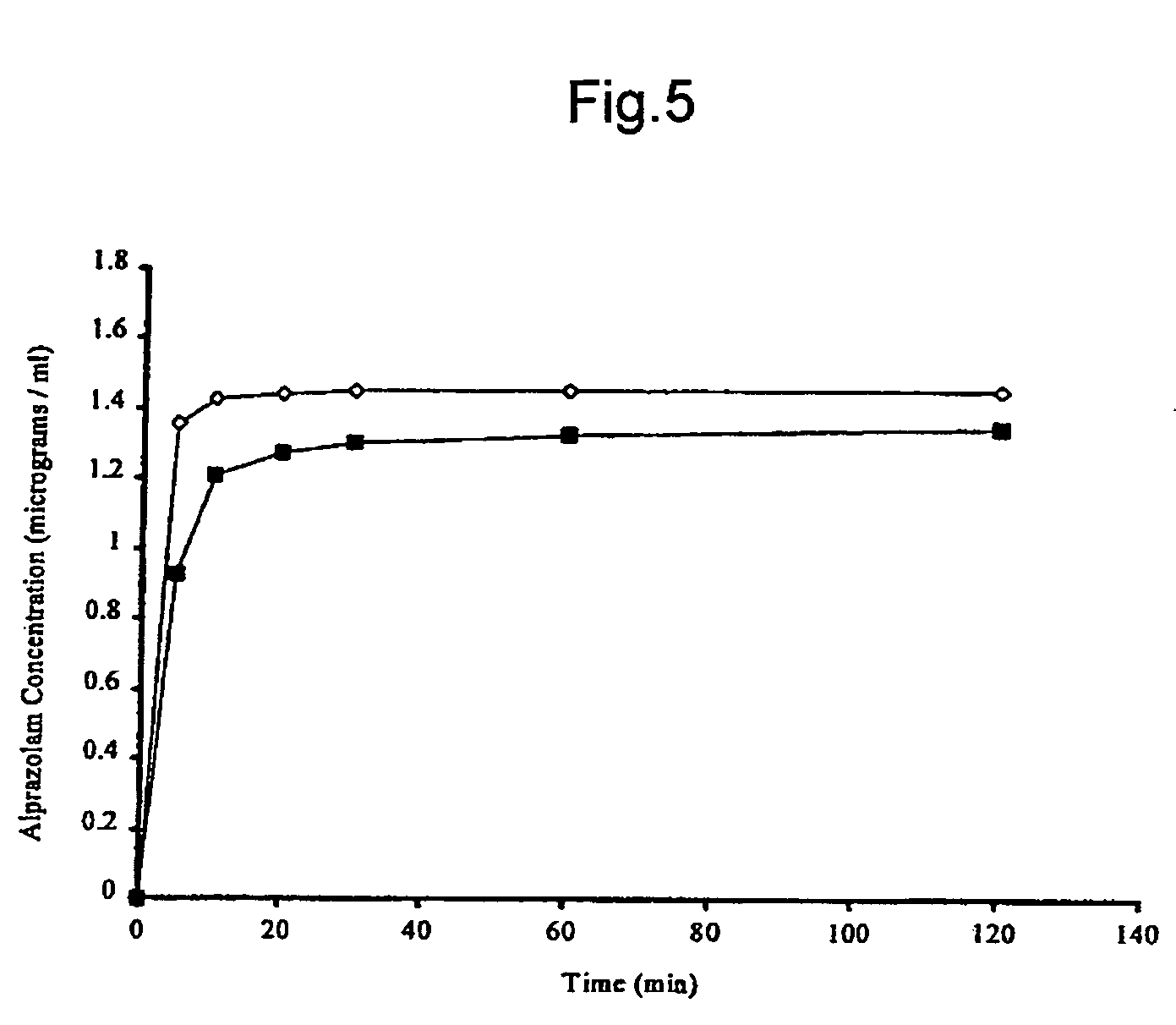
Alprazolam inclusion complexes and pharmaceutical composition thereof
A pharmaceutical composition an inclusion complex and methods for treating patients and preparing said complex disclosed for transmucosal delivery comprising an inclusion complex of (a) alprazolam and (b) a water soluble 2-hydroxypropyl-beta-cyclodextrin, and a pharmaceutically acceptable carrier therefor, wherein all the alprazolam is present in ring-closed form.
Owner:FARMARC NEDERLAND
Compound salvianic acid injection for treating cerebral vascular thrombosis diseases and preparation process thereof
InactiveCN102379864AImprove clinical efficacyQuality improvementPowder deliveryAntipyreticDiseaseCoronary heart disease
The invention aims to provide a compound salvianic acid injection which uses salvianic acid and paeonol as main components with high efficiency, advanced dosage form and stable preparation, as well as a preparation process thereof, and belongs to the field of biological pharmacy. The method comprises the following steps: extracting salvianic acid from salvia, and extracting paeonol from peony bark and enamelling with 2-hydroxypropyl-beta-cyclodextrin to prepare paeonol- hydroxypropyl-beta-cyclodextrin clathrate compound; weighting and mixing the salvianic acid, the paeonol clathrate compound and an excipient according to marked weights in the prescription, dissolving with injection water, filtering, and regulating the pH of the filtrate to be between 6.8 and 7.2; sterilizing and filtering, and diluting with aseptic injection water to the total designing solution; and packaging in a brown glass tube vial, and lyophilizing. The injection has the effects of activating blood circulation to remove stasis, and dredging collaterals and relieving pain, and is suitable for patients with cerebral vascular thrombosis diseases, such as coronary heart disease, angina pectoris, cerebral thrombosis, cerebral infarction and the like. The injection for adults is used for intramuscular injection or intravenous drip, and is dosed 1 to 5 vials one time, 1 to 2 times per day or as professionally prescribed.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Package plastic film
InactiveCN106883567AImprove tensile propertiesImprove water resistanceFlexible coversWrappersPolyamideSalicylic acid
The invention discloses a package plastic film which comprises the following raw materials in parts by weight: 20-35 parts of PC (polycarbonate), 25-40 parts of APET (amorphous polyester), 18-26 parts of PLA (polylactic acid), 4-6 parts of 2-hydroxypropyl-beta-cyclodextrin, 5-11 parts of ASA (acetyl salicylic acid) high glue powder, 7-18 parts of polyamide wax powder, 2-8 parts of 4-dimethylamino isoamyl benzoate, 5-18 parts of trehalose, 5-11 parts of diacetyl tartaric acid ester of mono(di) glycerides, 11-18 parts of tartrate, 11-18 parts of bisphenol A cyanate ester, 4-10 parts of polybutylene terephthalate, 2-5 parts of 3,5-diaminobenzoic acid, 5-11 parts of petroleum coke and 0.1-0.5 part of lithium amide. The package plastic film has good tensile capacity and water resistance since the raw materials are compounded to exert a synergistic effect; the production cost is low; a production technology is simple; the industrial production is facilitated; and the package plastic film can be manufactured into various package bags, and has a higher practical value and a high market popularization value.
Owner:HEFEI ZHIHUI LONGTUTENG INTPROP CO LTD
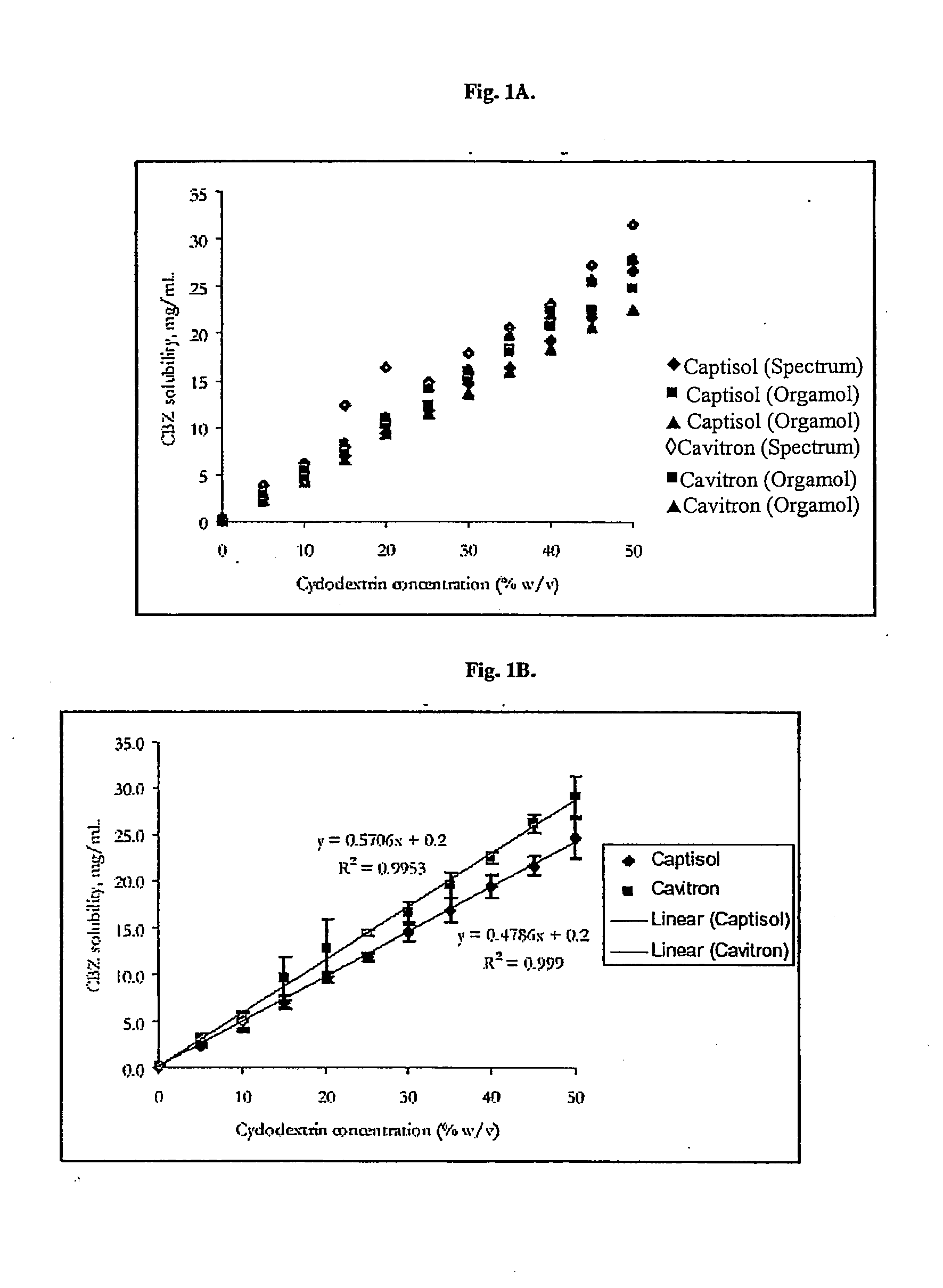
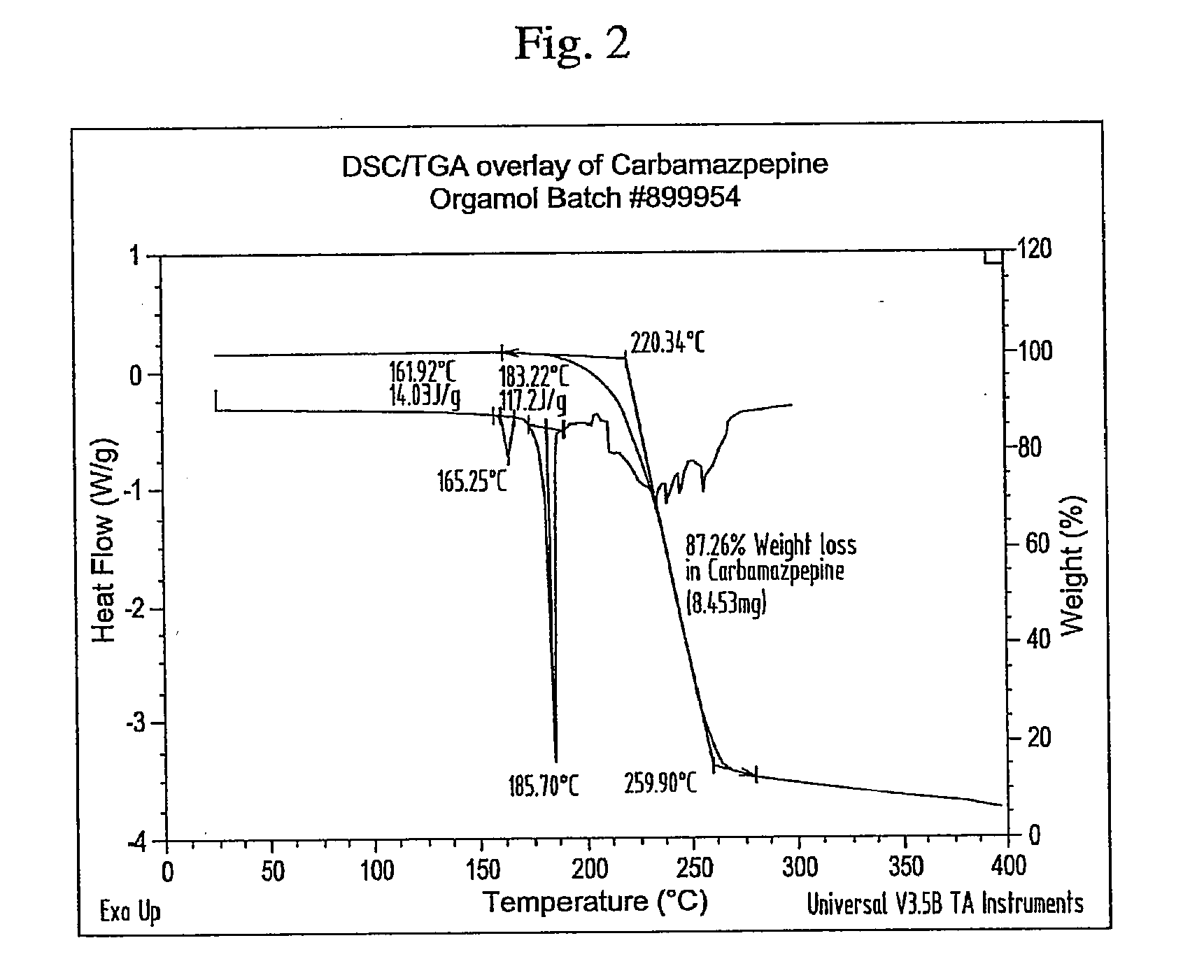
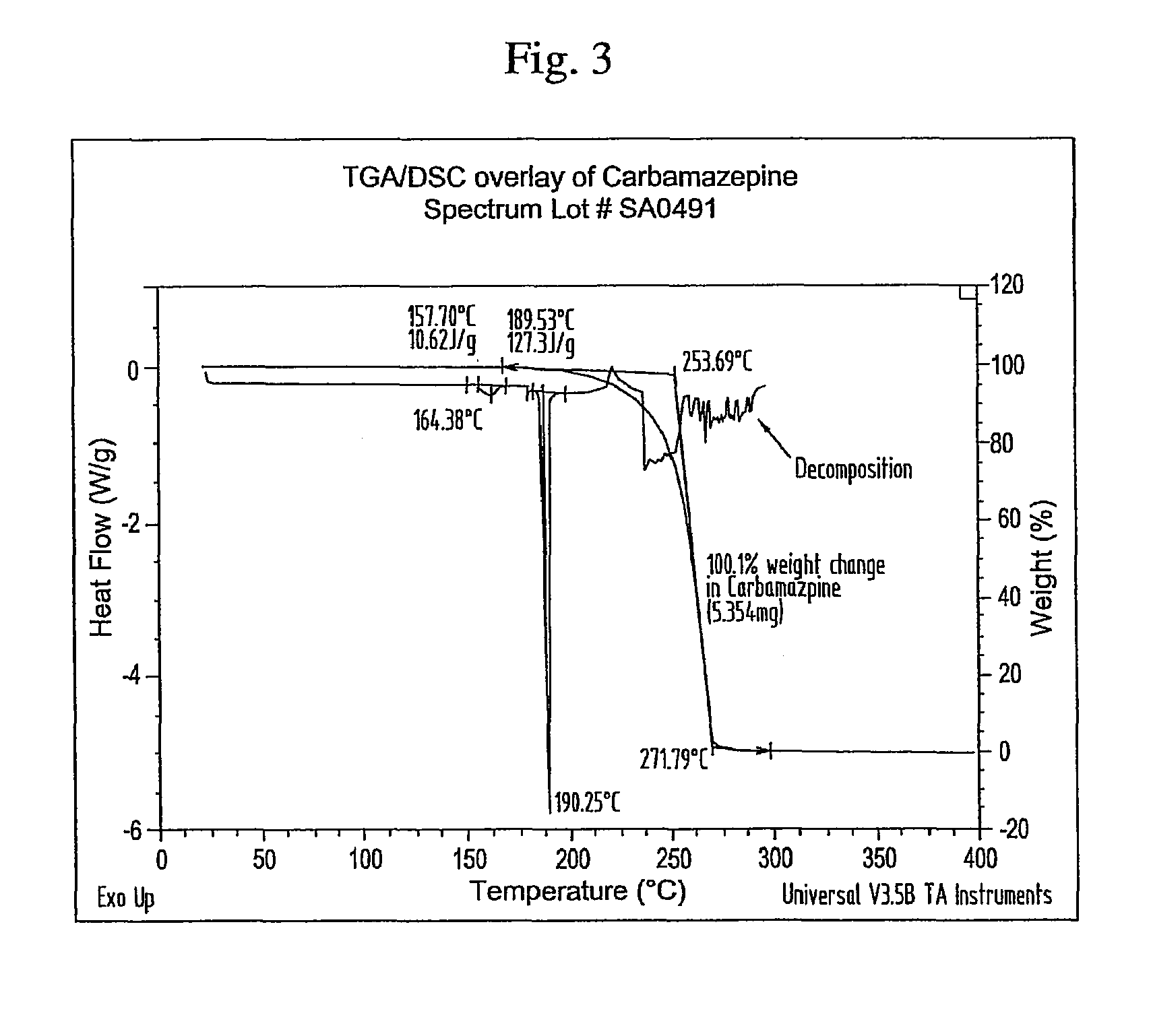
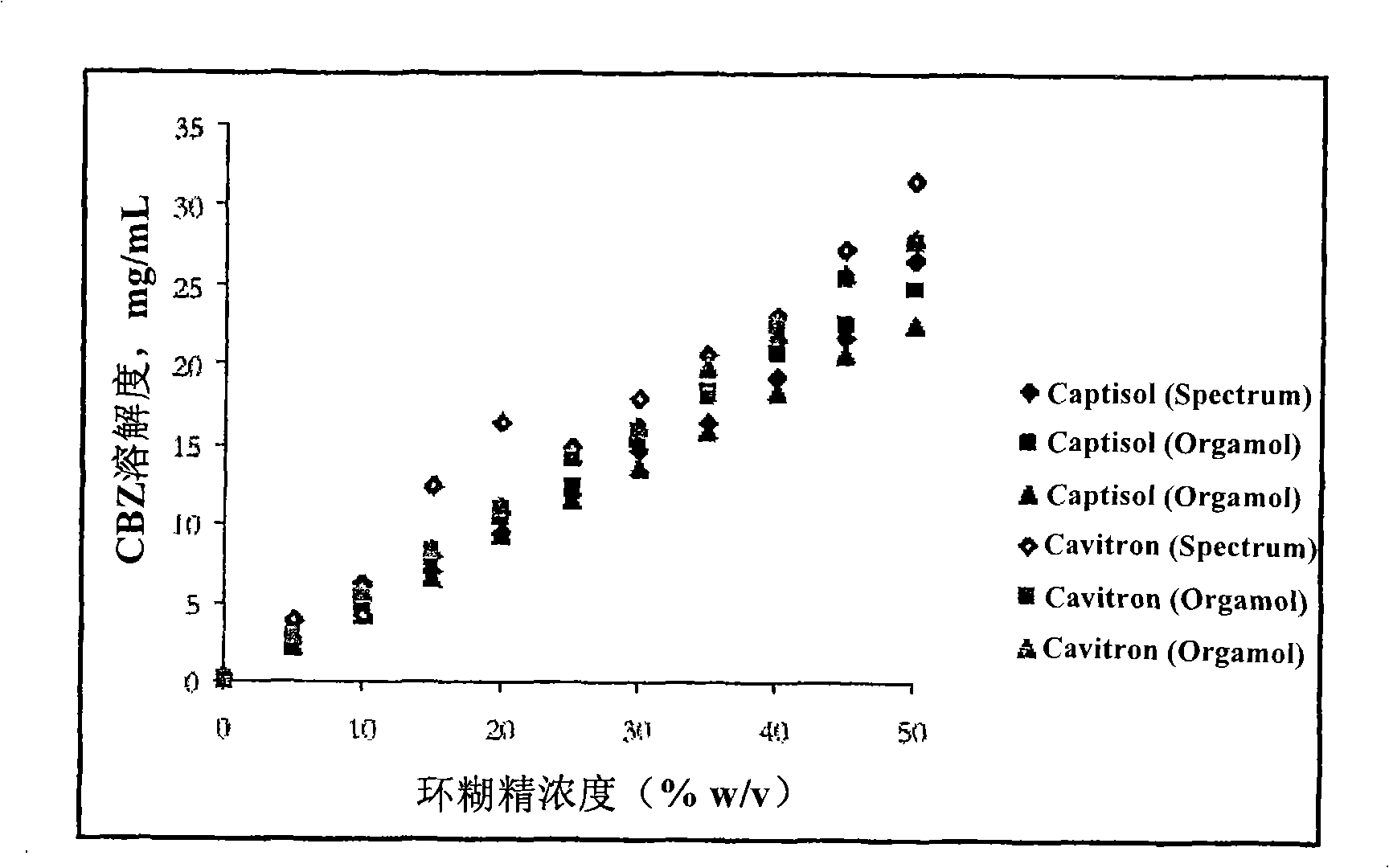
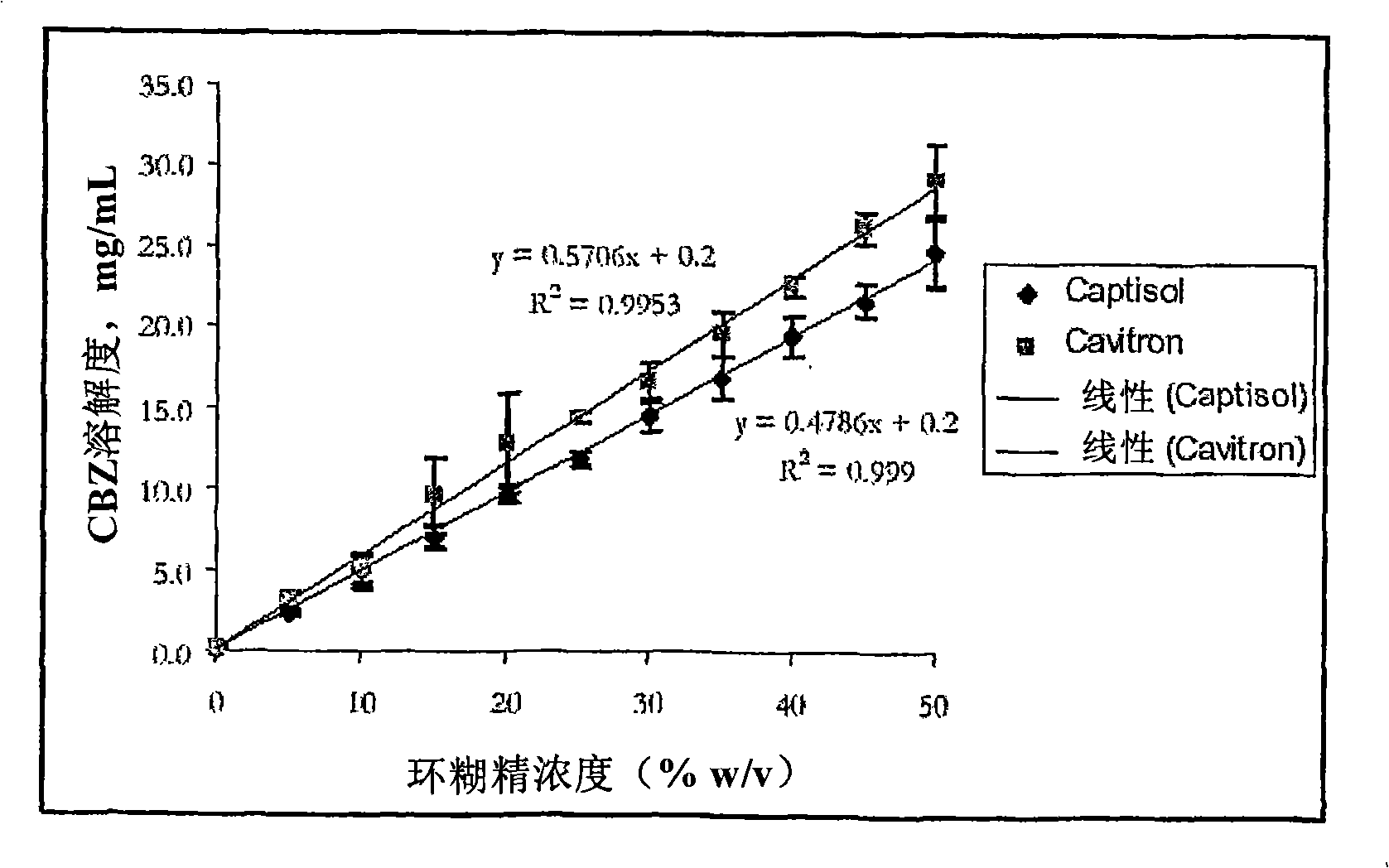
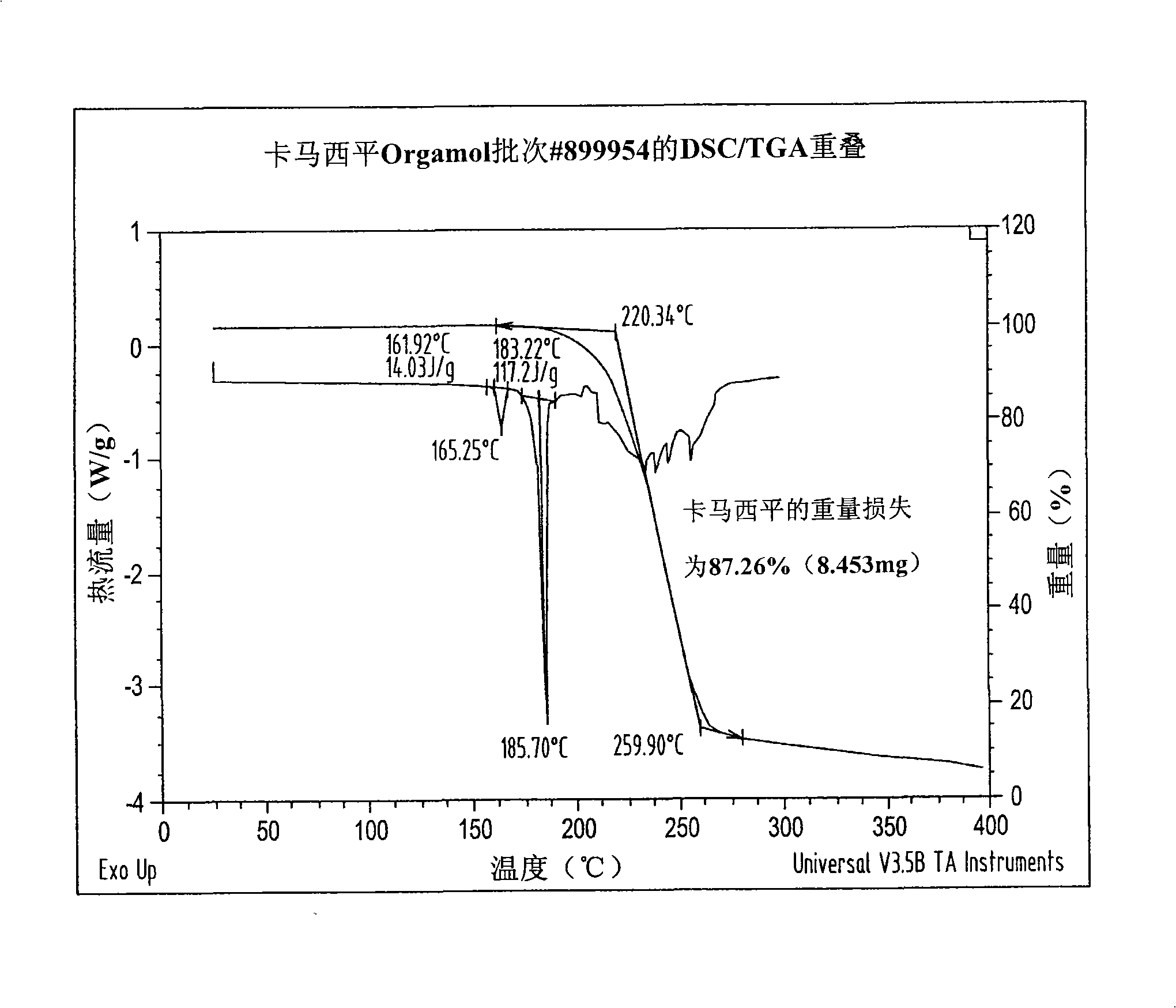
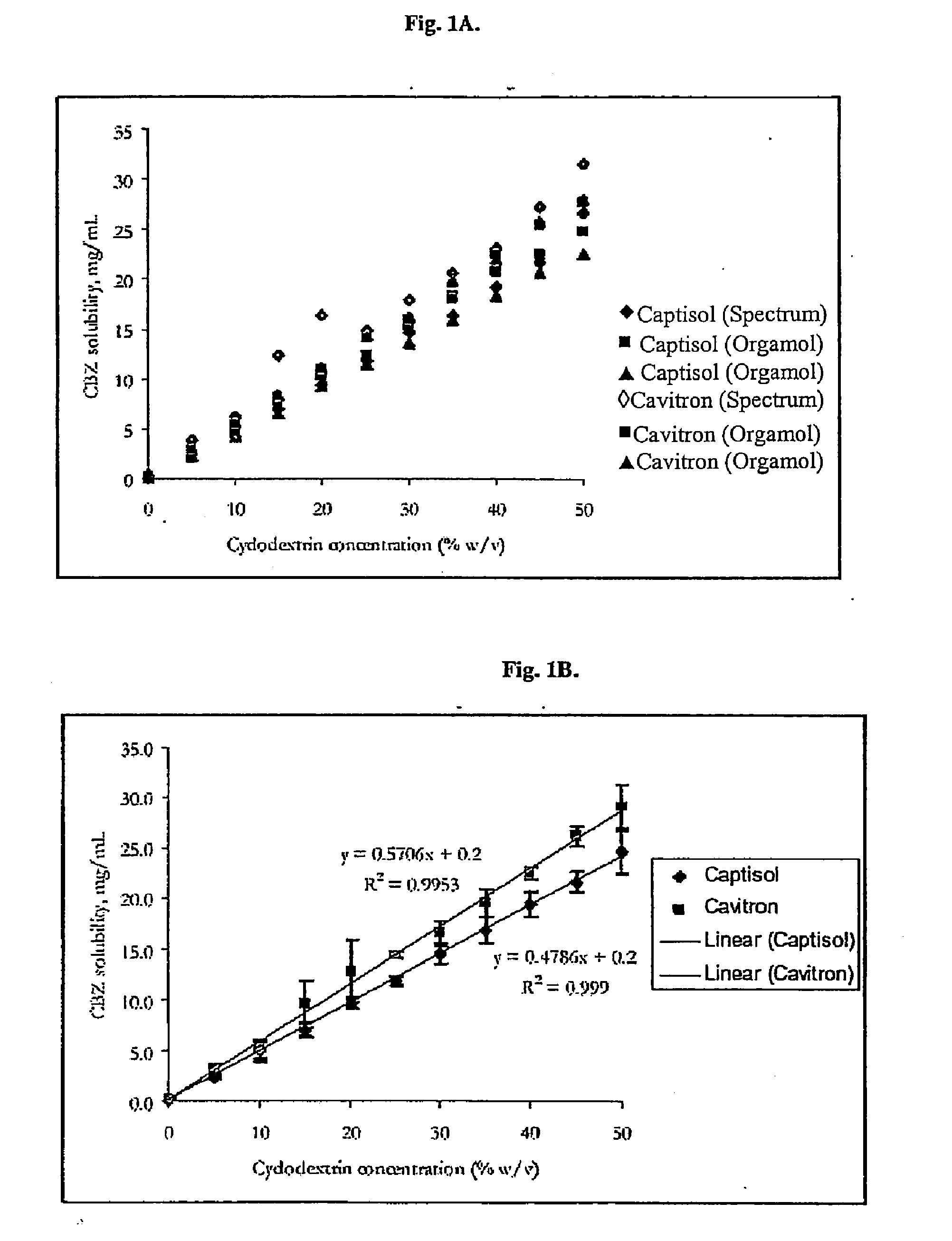
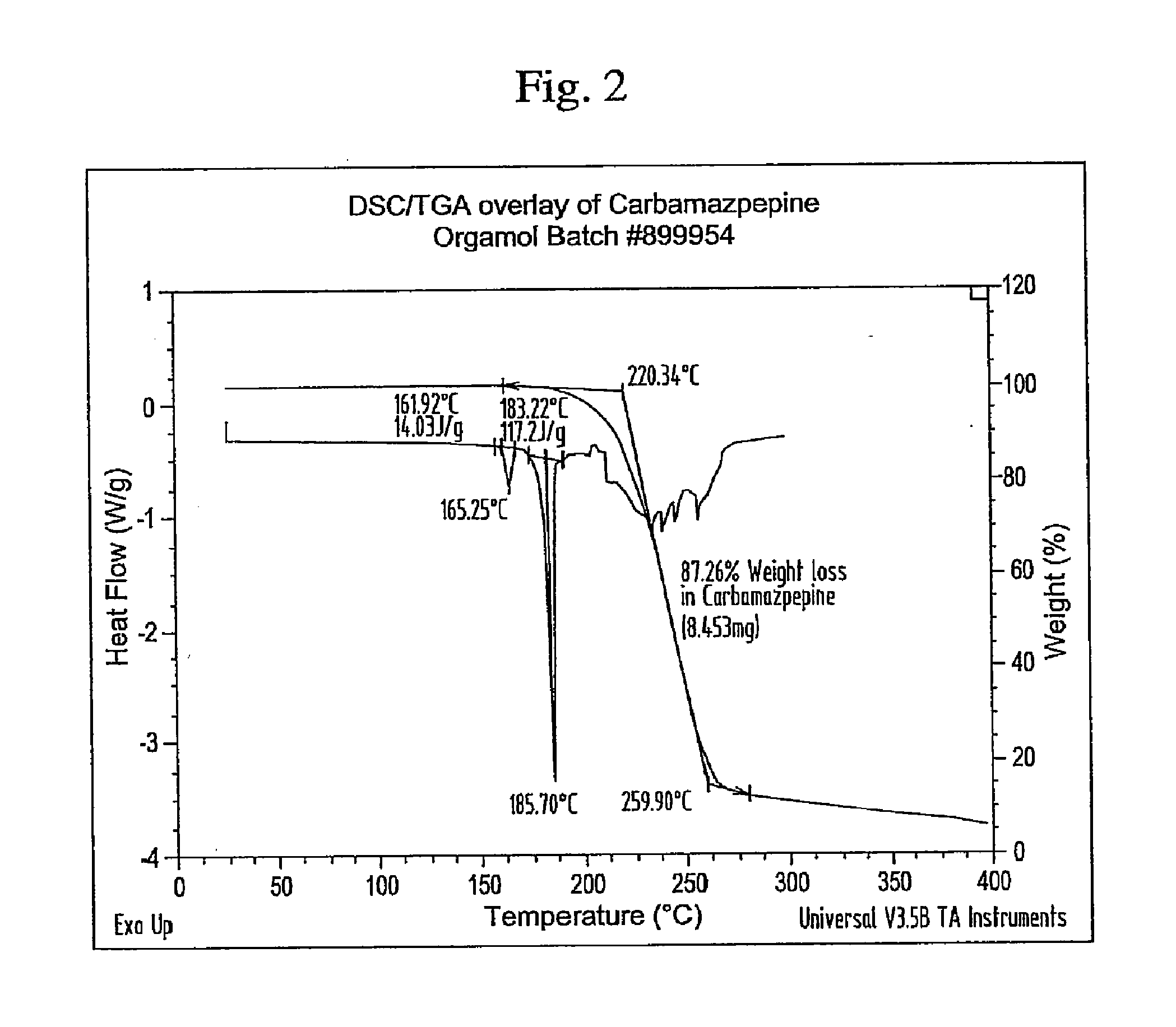
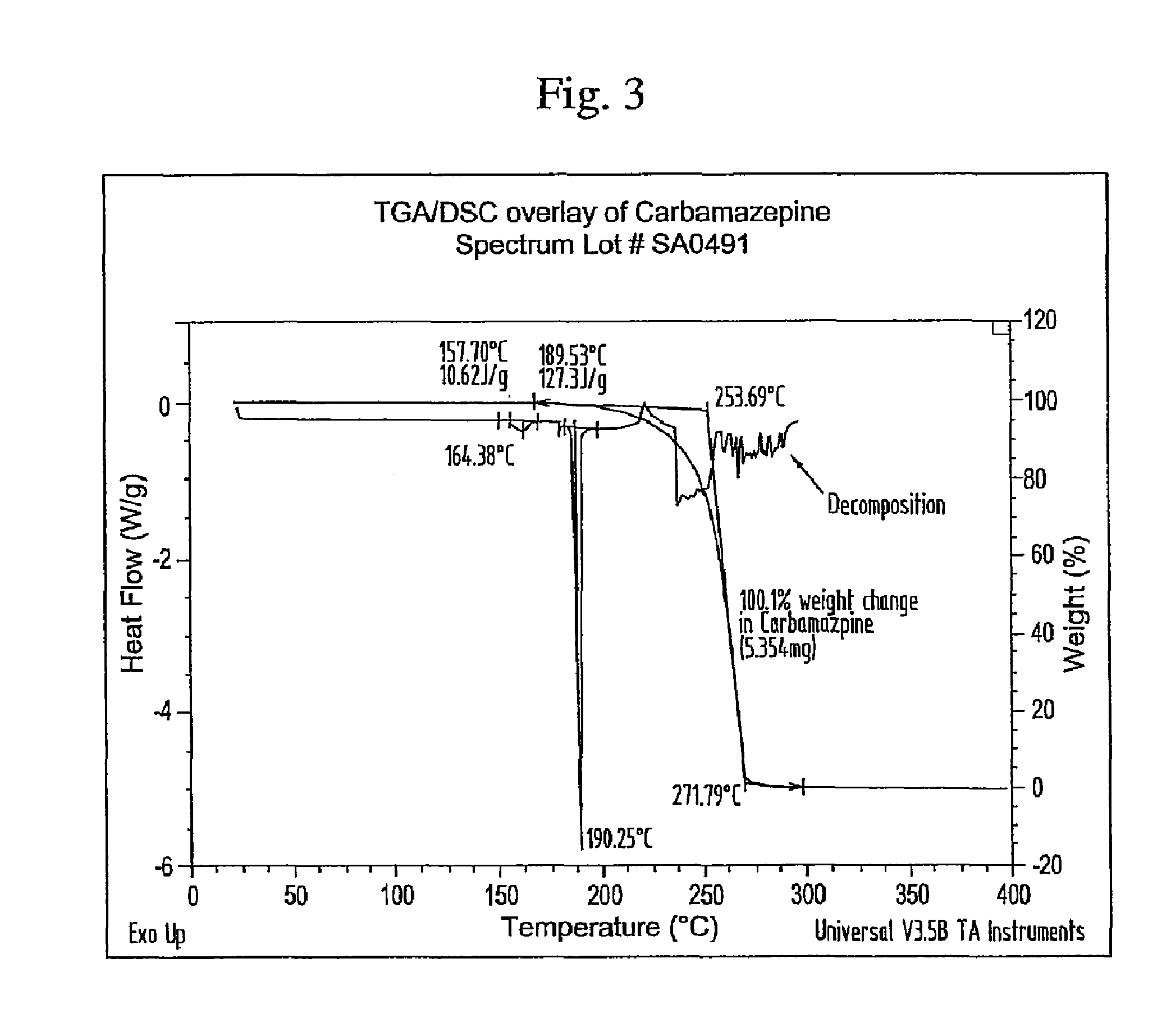
Novel parenteral carbamazepine formulation
The present invention is directed to a carbamazepine-cyclodextrin inclusion complex useful for the parenteral administration of carbamazepine. The carbamazepine-cyclodextrin inclusion complex is prepared by the admixture of a modified cyclodextrin and carbamazepine in a physiologically acceptable fluid. Modified cyclodextrins include 2-hydroxypropyl-beta-cyclodextrin and sulfoalkyl cyclodextrins. More particularly, the sulfoalkyl cyclodextrins are those described and disclosed in U.S. Pat. Nos. 5,134,127 and 5,376,645. A physiologically acceptable fluid includes sterile isotonic water, Ringer's lactate, D5W (5% dextrose in water), physiological saline, and similar fluids suitable for parenteral administration.
Owner:LUNDBECK LLC
Fireproof packaging film
InactiveCN109438827AFlame resistantGuaranteed mechanical propertiesFlexible coversWrappersLow-density polyethyleneLinear low-density polyethylene
The invention relates to the technical field of films, in particular to a fireproof packaging film. The fireproof packaging film is prepared from components in parts by mass as follows: 35-60 parts ofmetallocene linear low-density polyethylene, 4.5-7.5 parts of oxidized polyethylene wax, 2-6 parts of white master batch, 2-7 parts of polybutylene terephthalate, 3-8 parts of glass fiber, 2-5 partsof 2-hydroxypropyl-beta-cyclodextrin, 2-5 parts of hydroxypropyl acrylate, 4-6 parts of methacrylate, 0.3-0.5 parts of titanium dioxide, 2.1-3.8 parts of calcium carbonate powder, 7-9 parts of bentonite, 3-4 parts of a coupling agent, 4-8 parts of a flame retardant, 1-3 parts of a curing agent, 11-18 parts of tartrate, 1-3 parts of a compatilizer and 0.1-0.5 parts of lithium amide. According to the fireproof packaging film, the plastic film has flame retardance, original mechanical performance of the plastic film is kept, the use safety in building, aerospace and traffic industries and in lifesuch as the packaging material is guaranteed, and adverse conditions are prevented.
Owner:徐州金太阳包装材料有限公司
Novel parenteral carbamazepine formulation
The present invention is directed to a carbamazepine-cyclodextrin inclusion complex useful for the parenteral administration of carbamazepine. The carbamazepine-cyclodextrin inclusion complex is prepared by the admixture of a modified cyclodextrin and carbamazepine in a physiologically acceptable fluid. Modified cyclodextrins include 2-hydroxypropyl-beta-cyclodextrin and sulfoalkyl cyclodextrins. More particularly, the sulfoalkyl cyclodextrins are those described and disclosed in U.S. Patent Nos. 5,134,127 and 5,376,645. A physiologically acceptable fluid includes sterile isotonic water, Ringer's lactate, D5W (5 % dextrose in water), physiological saline, and similar fluids suitable for parenteral administration.
Owner:OVATION PHARMA
Ectomycorrhizal fungus complex microbial agent
The invention discloses an ectomycorrhizal fungus complex microbial agent. The ectomycorrhizal fungus complex microbial agent is prepared from 4-8 parts of complex ectomycorrhizal fungi, 2-6 parts of bacteria for dissolving phosphorus and potassium, 2-6 parts of compound mildew, 20-30 parts of straw powder, 20-30 parts of coconut velvet, 5-10 parts of ammonium molybdate, 2-6 parts of boracic acid, 3-8 parts of polyvinyl alcohol, 10-15 parts of 2-hydroxypropyl-beta-cyclodextrin, 10-15 parts of chitosan, 1-5 parts of growth hormone and 10-20 parts of olive fruit residues, wherein the complex ectomycorrhizal fungi are prepared by mixing pisolithus tinctorius, lactarius deliciosus and suillus bovinus; the bacteria for dissolving the phosphorus and potassium are bacillus megatherium and bacillus mucilaginosus; the compound mildew is a mixture of trichoderma longibrachiatum and streptomyces jingyangensis; various inoculants synergistically act to give play to a group joint action, and finally, a microbial community which is complex in composition, stable in structure and wide in function is formed. The plant and root growth can be promoted, the absorption of nutrient and water can be improved, it is achieved that the infection of a same host plant and different bacteria is diversified, nutrient absorption of the host plant is greatly improved, and the biomass is significantly improved.
Owner:SOUTHWEST UNIVERSITY
A kind of water-soluble two-photon polymerization initiator and assembly method and use
InactiveCN103864964BOvercome remaining deficienciesLow energy for laser processing3-dimensional image productionPhotosensitive materials for photomechanical apparatusOrganic solventLaser processing
The invention discloses a water-soluble two-photon polymerization initiator as well as assembling method and use thereof, the water-soluble two-photon polymerization initiator is assembled by host molecule 2-hydroxypropyl beta-cyclodextrin and guest molecule 2,7-di(4-pentoxy styrene)-anthraquinone, the molecular formula of the 2,7-di(4-pentoxy styrene)-anthraquinone is described by a formula I as shown in the specification. The water-soluble two-photon polymerization initiator is capable of initiating two-photon polymerization to process three-dimensional hydrogel in a water phase, the laser processing energy is lower in comparison with the processing energy (20-60mW) in the prior art, and the disadvantage of the residue of the organic solvent in the prior art is overcome.
Owner:TIANJIN UNIV
Ceftriaxone combinatorial drug
InactiveCN103908673AGood treatment effectPyrogenic reaction noAntibacterial agentsOrganic active ingredientsCurative effectBULK ACTIVE INGREDIENT
The invention provides a ceftriaxone combinatorial drug. The ceftriaxone combinatorial drug is characterized in that the ceftriaxone combinatorial drug is prepared from ceftriaxone, 2-hydroxypropyl-beta-cyclodextrin and tiopronin as active ingredients according to a weight part ratio of ceftriaxone, 2-hydroxypropyl-beta-cyclodextrin to tiopronin of 53-98: 5-11: 6-19. The invention also provides a preparation method of the ceftriaxone combinatorial drug. The ceftriaxone combinatorial drug is superior to the existing drug prepared by the prior art in safety, stability and curative effects and can be prepared by an energy-saving and eco-friendly preparation method.
Owner:邓学峰
Stretch-resistant and aging-resistant plastic film
The invention discloses a stretch-resistant and aging-resistant plastic film. The stretch-resistant and aging-resistant plastic film comprises, by weight, 50-60 parts of PVC (polyvinyl chloride) resin, 20-25 parts of polystyrene, 10-15 parts of disulfide carbamic acid ester, 10-15 parts of elastomers, 10-15 parts of bamboo charcoal powder, 3-7 parts of plasticizers, 5-9 parts of sodium alginate, 10-14 parts of carbon fibers, 5-10 parts of glass fibers, 3-5 parts of 2-hydroxypropyl-beta-cyclodextrin, 3-5 parts of nanometer titanium dioxide powder, 2-4 parts of nanometer tin antimony oxide powder, 1-3 parts of light stabilizers, 2-4 parts of natural antibacterial agents and 2-4 parts of plant oil. The stretch-resistant and aging-resistant plastic film has the advantages that the stretch-resistant and aging-resistant plastic film is economical, environmentally friendly, antistatic, stretch-resistant, aging-resistant, fog-resistant, safe and reliable and is wide in application, and the physiochemical properties of the stretch-resistant and aging-resistant plastic film can be effectively controlled while high light permeability of the stretch-resistant and aging-resistant plastic film is guaranteed; excellent antibacterial, weather-resistant, stable, heat-insulation, anti-ultraviolet and self-cleaning effects can be realized by the stretch-resistant and aging-resistant plastic film, and the like.
Owner:安徽省天乐塑业有限公司
Application of 2-hydroxypropyl-beta-cyclodextrin to the preparation of drug for treatment of X-linked adrenoleukodystrophy
ActiveCN104857014AReduce VLCFA levelsReduce behavioral symptomsOrganic active ingredientsMetabolism disorderPlasma total cholesterolLong chain fatty acid
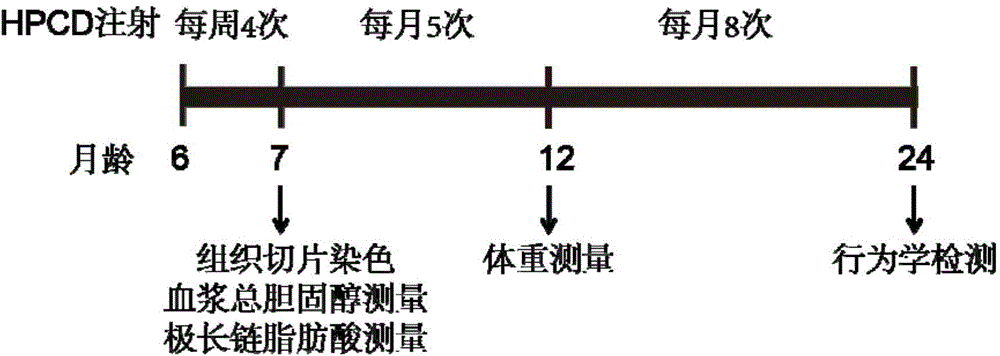
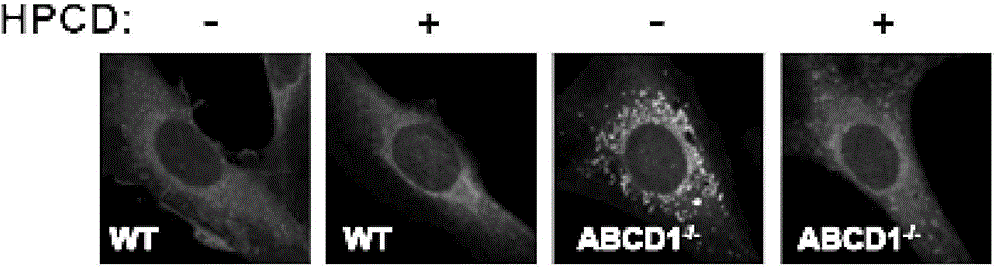
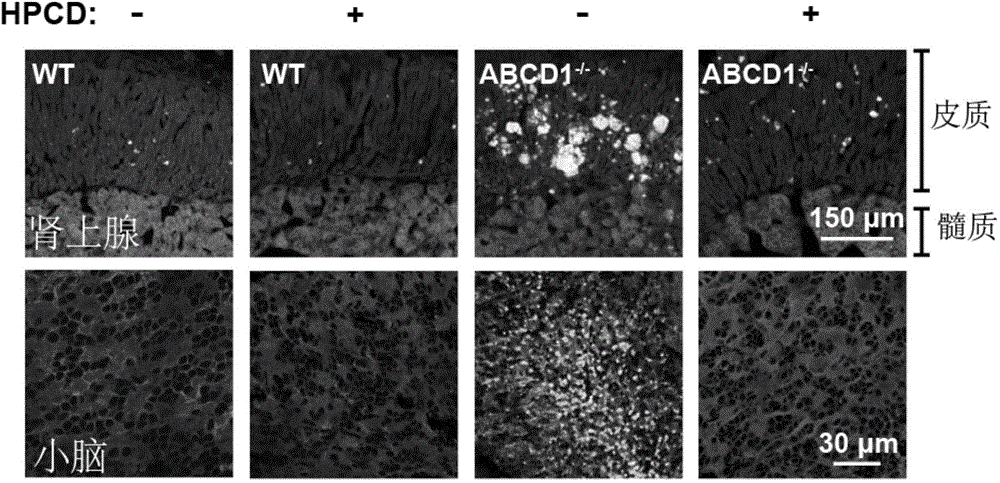
The present invention discloses application of a 2-hydroxypropyl-beta-cyclodextrin (HPCD) to the preparation of the drug for the treatment of X-linked adrenoleukodystrophy (X-ALD), and belongs to the biomedical field. The invention is as below: injecting 2-hydroxypropyl-beta-cyclodextrin into the wild mice and X-ALD model mice (ABCD1 knockout), conducting Filipin staining on the cell, adrenal gland and cerebellum tissue slices of the mice injected with HPCD; measuring the plasma total cholesterol, very long chain fatty acids and weight, and conducting behavioral tests. The results show that HPCD can make cholesterol levels if plasma, cerebellum and adrenal drop into the normal range, also reduce the VLCFA levels, and mitigate the behavior abnormalities caused by neurodegeneration of X-ALD mice. The results show that HPCD can treat X-ALD and can be applied to the drug for treatment of X-ALD.
Owner:WUHAN UNIV
Health-care medicines rich in SOD and preparation method of health-care medicines
InactiveCN109966478AGood health effectImprove immunityPeptide/protein ingredientsAntinoxious agentsDiseaseAngelica sinensis
The invention discloses health-care medicines rich in SOD and a preparation method of the health-care medicines rich in SOD. The health-care medicines rich in SOD comprise the following components inparts by weight of 1-3 parts of superoxide dismutase, 0.2-0.4 part of sialic acid, 3-5 parts of 2-hydroxypropyl-beta-cyclodextrin, 0.02-0.03 part of natural vitamin E, 0.01-0.02 part of natural vitamin A, 30-40 parts of radix angelica sinensis, 10-12 parts of collagen liquid, and 20-30 parts of sugar-cane juice. The health-care medicines rich in SOD are high in health-care effects, and have the efficacy of improving immunity, strengthening bodies, regulating functions, preventing disease propagating and the like. Various effective components are in synergistic effects, so that the efficacy ofresisting oxidation and resisting ageing of products can be improved.
Owner:上海梓惜生物技术有限公司
Urapidil hydrochloride injection and preparation method thereof
ActiveCN104173281AHigh sterilization temperatureHas industrial valueOrganic active ingredientsPharmaceutical delivery mechanismDrugs preparationsAmino acid
The invention relates to urapidil hydrochloride injection and a preparation method thereof, and belongs to the technical field of a drug preparation. The injection comprises the following raw materials of urapidil hydrochloride, an amino acid complex salt and 2-hydroxypropyl-beta-cyclodextrin at the mass ratio of 1 to (1-1.5) to (4-7). The urapidil hydrochloride injection has the characteristics of being easy to store and transport, sterilization-resistant, good in stability, not easy to go bad, and relatively convenient in clinical application.
Owner:HEBEI YIPIN PHARMA
Novel parenteral carbamazepine formulation
Owner:LUNDBECK PHARMA LLC
Preparation method of biological natural preservative and application thereof in sturgeon caviar
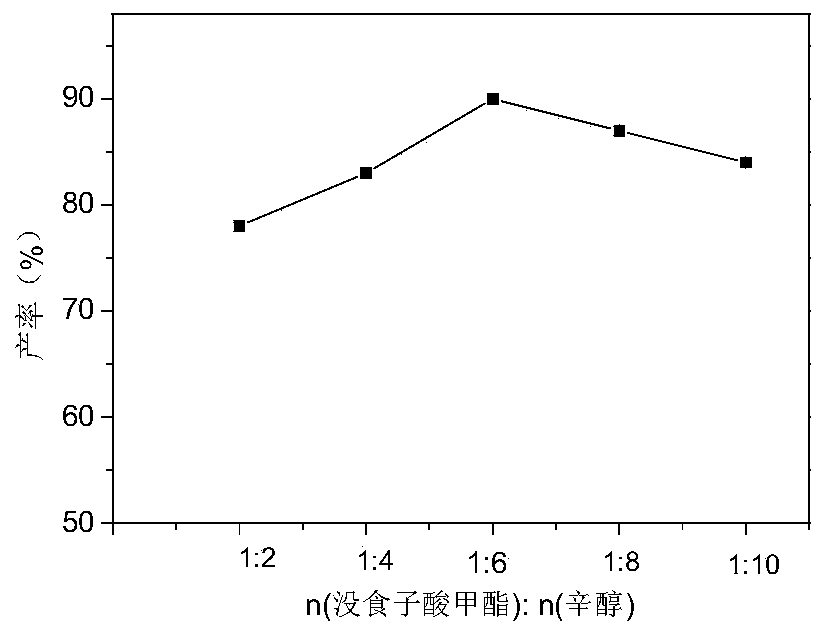
The invention relates to a preparation method of a biological natural preservative and application thereof in sturgeon caviar. An octyl gallate / 2-hydroxypropyl-beta-cyclodextrin encapsulation naturalpreservative is disclosed. The octyl gallate and 2-hydroxypropyl-beta-cyclodextrin are encapsulated according to a molar ratio of 1:0 .5-1.5. A method for preparing the octyl gallate by an enzymatic method in a deep eutectic solvent is also disclosed. In addition, the application of the preservative in the preservation of the sturgeon caviar is also disclosed. The processed caviar not only maintains the efficacy for a long time, but also effectively reduces the storage cost. The preservative is tasteless, non-toxic and edible, and the components are safe and environmentally friendly. The watersolubility and the antibacterial effect of the octyl gallate are improved, and the pollution of pathogenic bacteria to aquatic products during storage and transportation is effectively inhibited. Theshelf life can be effectively prolonged. The application range of the octyl gallate as the preservative in the food field is expanded.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Sustained-release mosquito repellent sheet
InactiveCN108077328AImprove mosquito repellent effectImprove the mixing effectBiocidePest repellentsSustained Release CapsuleUltraviolet
The invention discloses a sustained-release mosquito repellent sheet. The sustained-release mosquito repellent sheet comprises, by weight, 80 parts of EVA (ethylene-vinyl acetate) and 25 parts of sustained-release capsules. The sustained-release capsules comprise, by weight, 30 parts of plant essential oil, 5 parts of eucalyptus essential oil, 200 parts of acrylic acid, 10 parts of a cross-linkingagent, 1 part of an initiator, 20 parts of a modified molecular sieve and 30 parts of 2-hydroxypropyl-beta-cyclodextrin, and the modified molecular sieve comprises, by molar ratio, 2 parts of tetrabutyl orthosilicate, 1 part of tetraethyl orthosilicate, 0.1 part of tetra(2-fluoroethyl) silicate, 0.1 part of tetramethylammonium silicate, 0.1 part of cobaltous silicate, 1 part of N-bromomethylene-N,N-dimethylammonium bromide and 0.2 part of (1-hexyl) trimethyl ammonium bromide; the plant essential oil is selected from at least one of mono-terpenol essential oil, monoterpene essential oil, aldehyde essential oil and ester essential oil. The sustained-release mosquito repellent sheet never fails even if ultraviolet rays irradiate on the sustained-release mosquito repellent sheet for a long time.
Owner:ZHEJIANG SEMIR GARMENT CO LTD +1
Functionalized montmorillonite and application thereof in fruit-vegetable detergent
PendingCN109576076AStrong decontaminationEfficient removalInorganic/elemental detergent compounding agentsNon-ionic surface-active compoundsWaxPesticide residue
The invention discloses functionalized montmorillonite and application thereof in a fruit-vegetable detergent. The functionalized montmorillonite is prepared by acidifying montmorillonite and modifying through a modifier. The modifier is one of beta-cyclodextrin, 2, 6-dimethyl-beta-cyclodextrin and 2-hydroxypropyl-beta-cyclodextrin. Compared with the prior art, the functionalized montmorillonite has the advantages that the fruit-vegetable detergent prepared by applying the functionalized montmorillonite is rich in natural plant extract, high in detergency and capable of effectively removing pesticide residue and fruit wax on the surfaces of fruits and vegetables, can be used for cleaning fruits and vegetables and infant articles like feeding bottles and toys and is warm, nonirritant, healthy, environment-friendly and free of damage to human body.
Owner:刘岱军
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com