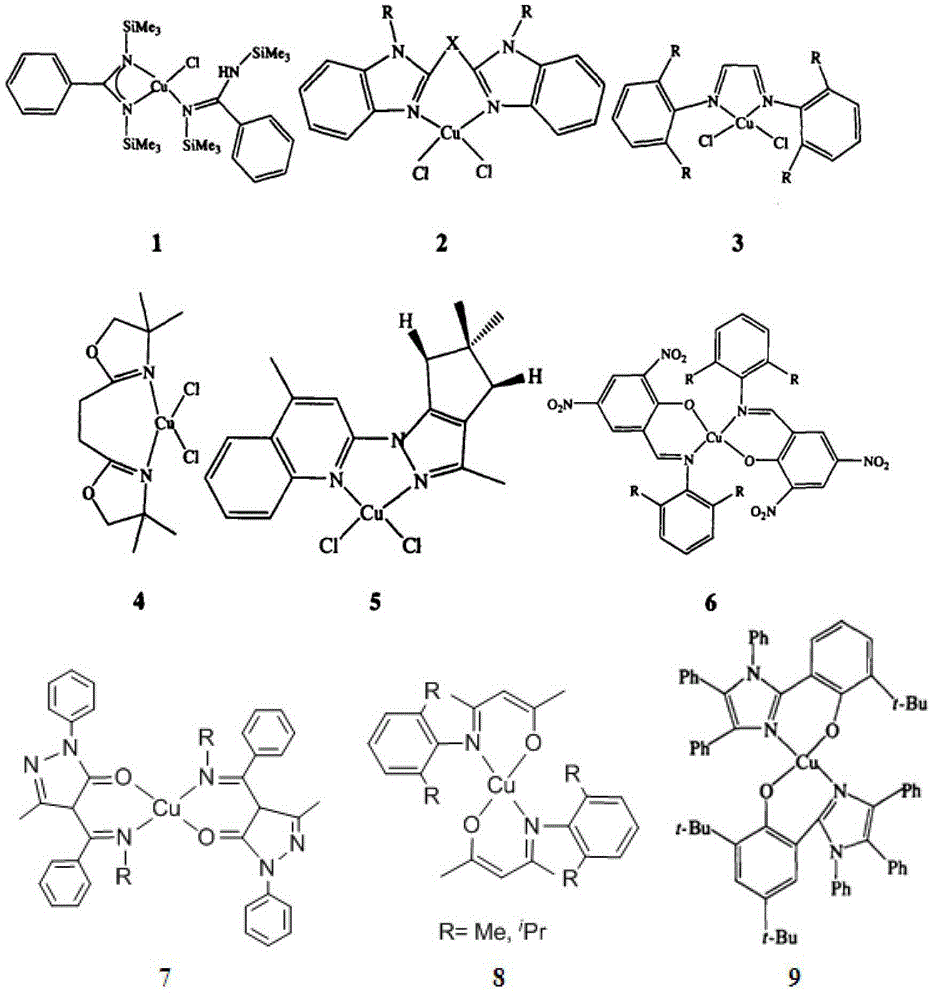
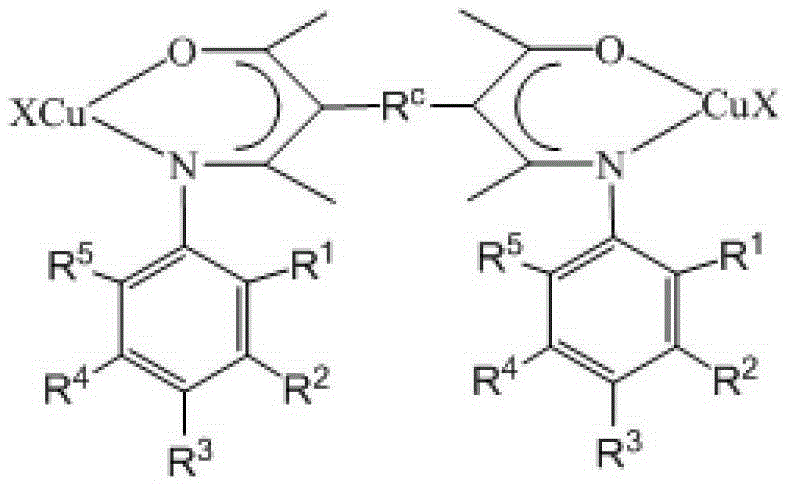
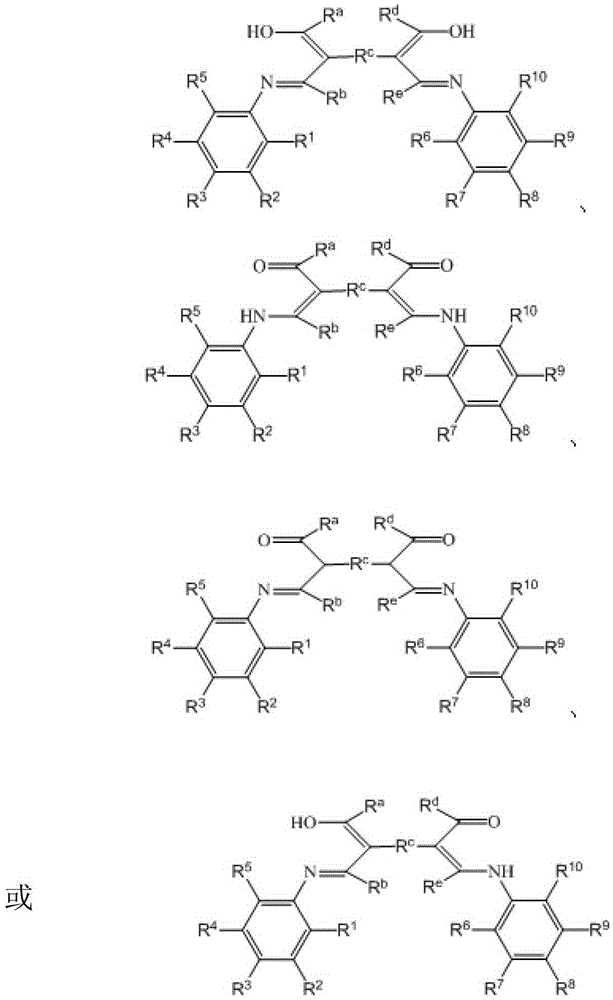
β-ketimine-coordinated binuclear copper complex, preparation method and use
A technology of copper complexes and ketimines, which is applied in the direction of copper organic compounds, can solve the problems of inability to catalyze the polymerization of styrene and butadiene, and the low catalytic activity of ethylene polymerization, and achieve simple synthesis methods, high activity and low reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of Binuclear Copper Complex 1 Coordinated by β-Ketimine (hereinafter referred to as Cu1)
[0033] Take the copper complex of 0.412g (1mmol) of ligand L1 and 0.52g (2mmol) of diacetylacetone in a round bottom flask, add 30ml of methanol, and heat to reflux for 5 hours. The reaction was stopped, and after the solution was cooled, the solvent methanol was distilled off under reduced pressure to obtain a crude product, which was then recrystallized with toluene to obtain 0.61 g of a black solid product with a yield of 82%. Elemental analysis (C 34 h 44 Cu 2 f 2 N 2 o 6 ): Theoretical value (%): C, 55.05; H, 5.98; N, 3.78. Test value (%): C, 55.70; H, 5.29; N, 4.20.
[0034]
[0035] L1Cu1
Embodiment 2
[0037] Synthesis of Binuclear Copper Complex 2 Coordinated by β-Ketimine (hereinafter referred to as Cu2)
[0038]
[0039] L2Cu2
[0040] Take 1mmol of ligand L2 and 2mmol of [Cu(NH 3 ) 4 ]Cl 2 Place in a round bottom flask, add 50ml of methanol, and heat to reflux for 6 hours. The reaction was stopped, and after the solution was cooled, the solvent methanol was distilled off under reduced pressure to obtain a crude product, which was then recrystallized with toluene to obtain a black solid with a yield of 76%. Elemental analysis (C 34 h 56 Cl 2 Cu 2 N 8 o 2 ): Theoretical value (%): C, 50.61; H, 7.00; N, 13.89. Test value (%): C, 50.52; H, 7.30; N, 13.67.
Embodiment 3
[0042] Synthesis of Binuclear Copper Complex 3 Coordinated by β-Ketimine (hereinafter referred to as Cu3)
[0043] Take 1 mmol of ligand L3 and 2 mmol of benzylcopper complex and put them in a round bottom flask, add 30 ml of dichloromethane, and heat to reflux for 3 hours. The reaction was stopped, and after the solution was cooled, the solvent dichloromethane was distilled off under reduced pressure to obtain a crude product, which was then recrystallized with toluene to obtain a black solid product with a yield of 62%. Elemental analysis (C 39 h 37 Cu 2 f 3 N 2 o 2 ): Theoretical value (%): C, 62.47; H, 4.97; N, 3.74. Test value (%): C, 62.70; H, 4.79; N, 3.50.
[0044]
[0045] L3Cu3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com