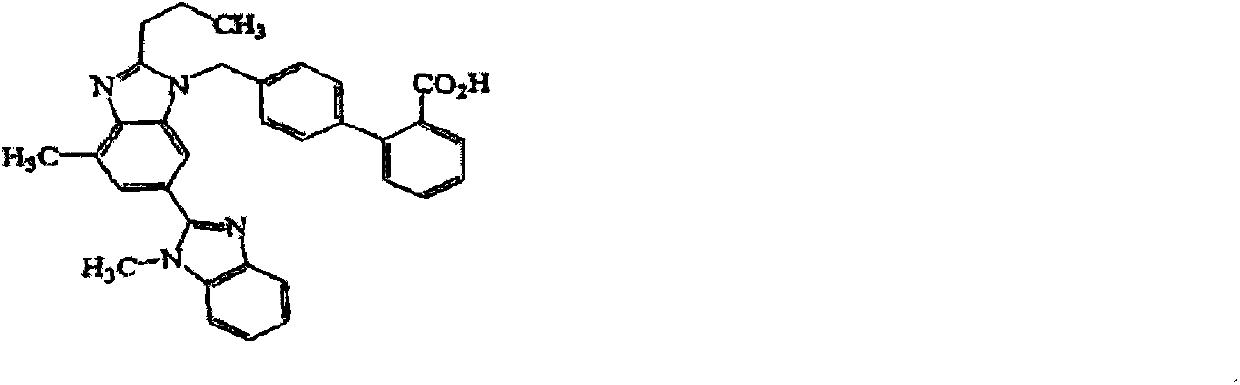
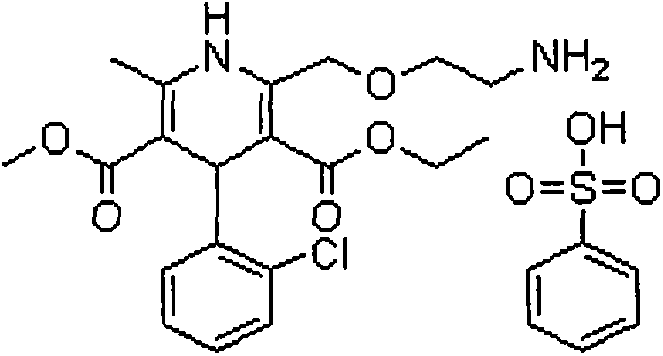
Oral tablet containing telmisartan and amlodipine besylate and preparation method thereof
A technology of amlodipine besylate and telmisartan, applied in the field of medicine, can solve problems such as time-consuming and laborious spray drying method, and achieve the effects of reducing production process steps, reducing production cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
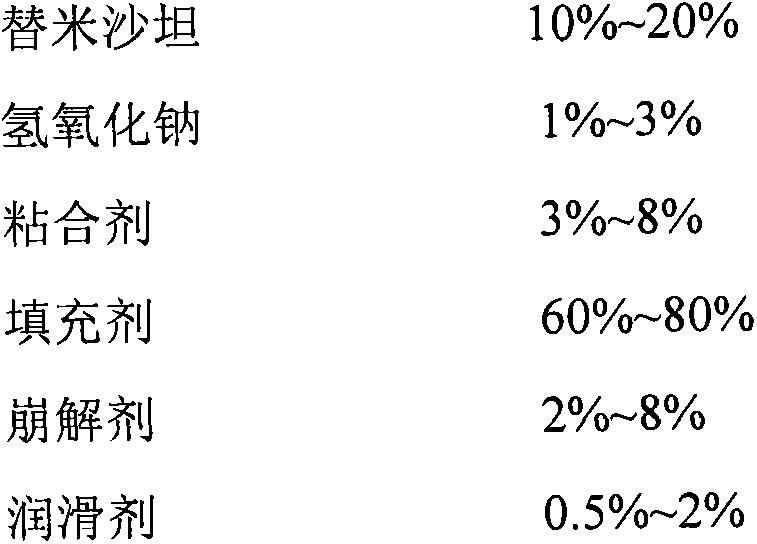
[0029]
[0030] The preparation method is as follows: dissolve telmisartan and alkaline reagent in water to make a uniform solution for later use; sieve and mix the filling agent, disintegrating agent and binder in the telmisartan tablet core and place it in a fluidized bed In the process, spray the above-mentioned uniform solution to granulate, and dry; sieve and granulate the dried granules, add the added pharmaceutical excipients and mix evenly, and then perform tablet compression to obtain telmisartan tablet cores; After uniform dispersion in the coating solution, the telmisartan tablet core is coated, and after the coating is completed, it is dried to obtain the telmisartan amlodipine tablet.
[0031] According to dissolution measurement method (Chinese Pharmacopoeia 2010 edition appendix XC second method), pH7.5 damping fluid is as dissolution medium, and rotating speed is 75m, to embodiment 1 sheet and import telmisartan amlodipine sheet (manufacturer: Germany Boehri...
Embodiment 2
[0046]
[0047] The preparation method is as follows: dissolve telmisartan and alkaline reagent in water to make a uniform solution for later use; sieve and mix the filling agent, disintegrating agent and binder in the telmisartan tablet core and place it in a fluidized bed In the process, spray the above-mentioned uniform solution to granulate, and dry; sieve and granulate the dried granules, add the added pharmaceutical excipients and mix evenly, and then perform tablet compression to obtain telmisartan tablet cores; After uniform dispersion in the coating solution, the telmisartan tablet core is coated, and after the coating is completed, it is dried to obtain the telmisartan amlodipine tablet.
[0048] According to dissolution measurement method (Chinese Pharmacopoeia 2010 edition appendix XC second method), pH7.5 damping fluid is as dissolution medium, and rotating speed is 75m, to embodiment 1 sheet and import telmisartan amlodipine sheet (manufacturer: Germany Boehri...
Embodiment 3
[0062]
[0063]
[0064] The preparation method is as follows: dissolve telmisartan and alkaline reagent in water to make a uniform solution for later use; sieve and mix the filling agent, disintegrating agent and binder in the telmisartan tablet core and place it in a fluidized bed In the process, spray the above-mentioned uniform solution to granulate, and dry; sieve and granulate the dried granules, add the added pharmaceutical excipients and mix evenly, and then perform tablet compression to obtain telmisartan tablet cores; After uniform dispersion in the coating solution, the telmisartan tablet core is coated, and after the coating is completed, it is dried to obtain the telmisartan amlodipine tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com