Process for producing fatty alcohols from fatty acids
A technology for fatty alcohols and fatty acids, applied in the field of preparing fatty alcohols, can solve problems such as loss of feed efficiency, and achieve the effects of maximum loss and minimal loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
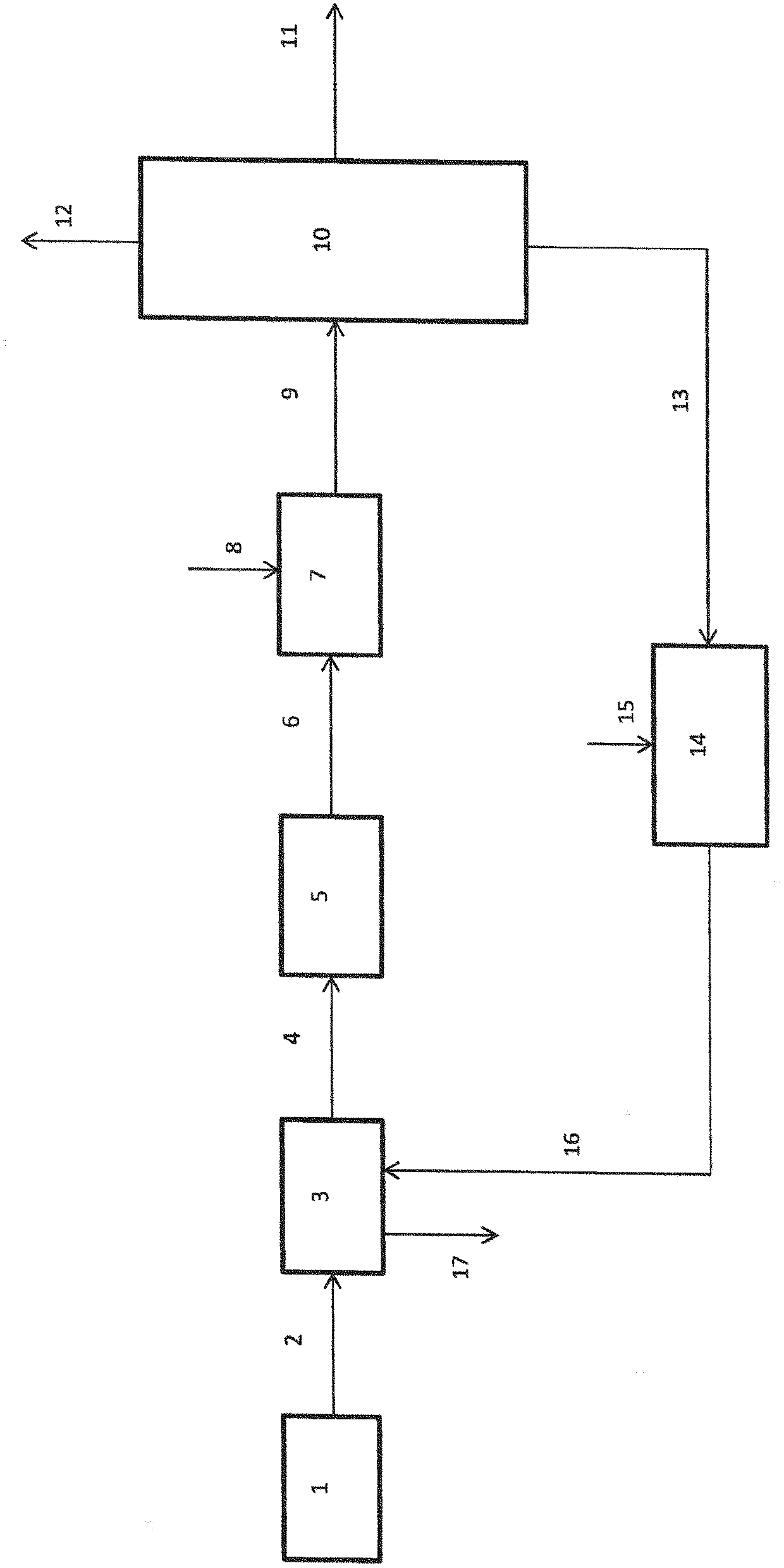
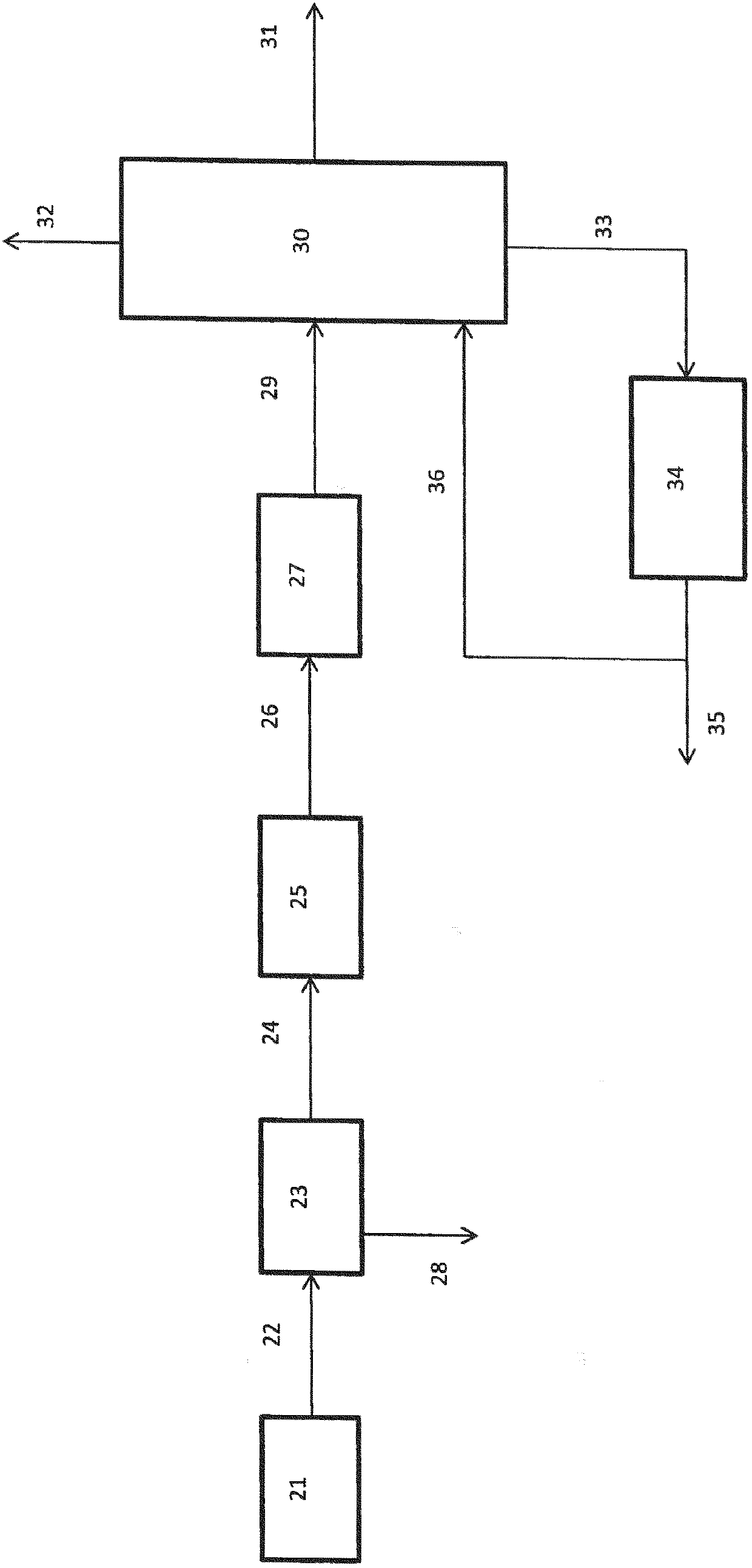
Image
Examples
Embodiment 1
[0068] A standard 300ml autoclave was assembled with a bottom drain point and a sample bomb to allow addition of liquid at operating temperature and pressure. The static catalyst basket was also shaped to allow the solid catalyst titanium dioxide (TiS) supported on silica, referred to as Catalyst-1, to be held within the liquid contents of the autoclave.
[0069] 2g of Catalyst-1 was added to the basket and 200ml of 1-dodecanol (157.9g ex Aldrich) was added to the container. The autoclave was heated to 215°C for over 1 hour and 2 g of methyl laurate (ex Aldrich) was added through the sample reservoir which had been pressurized with nitrogen. Excess nitrogen pressure was then vented, and the autoclave was sealed. Then, after the first small purge was collected, the contents were sampled over time through the bottom drain valve.
Embodiment 2 to 6
[0071] Then, the test was repeated using the same catalyst loading. Example 2 was the same as Example 1 to check for total inactivation. Examples 3 and 4 used double the methyl laurate concentration, Example 5 had the agitation rate reduced to check for weight transfer limitations, and Examples 4, 5 and 6 had 4 wt% added to the initial tank contents Methanol. Practice 6 can be performed under pressure (52 psig).
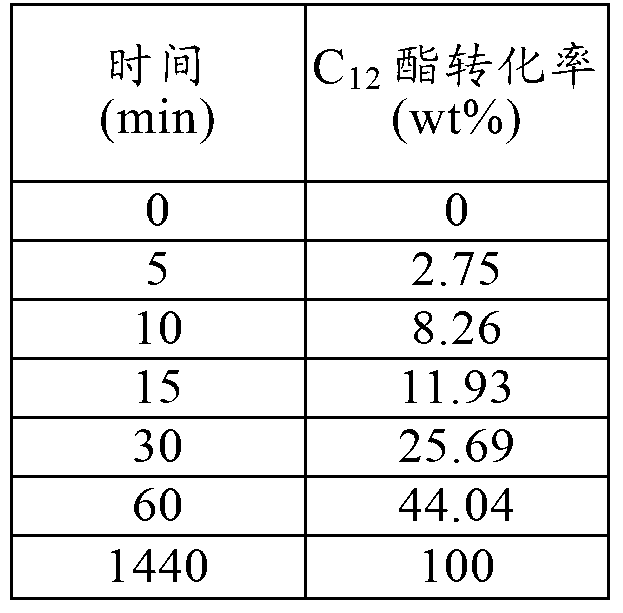
[0072] Tables 1-6 show the progression of the reaction over time.
[0073] Table 1 - Example 1
[0074]
Embodiment 2
[0076]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com