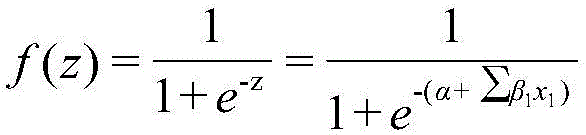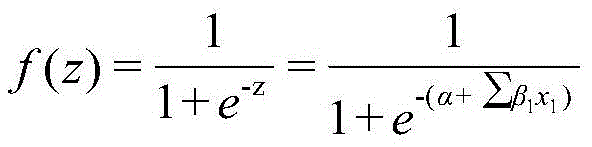Prediction of Biodegradability of Organic Chemicals Using Logistic Regression Method
A technology of organic chemicals and biodegradability, applied in the field of ecological risk assessment and testing strategies, can solve problems such as unfavorable model application and mechanism interpretation, inability to extract prediction rules, poor comprehensibility, etc., to achieve easy analysis and understanding and practical application, Good fitting effect, effect of transparent prediction rules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
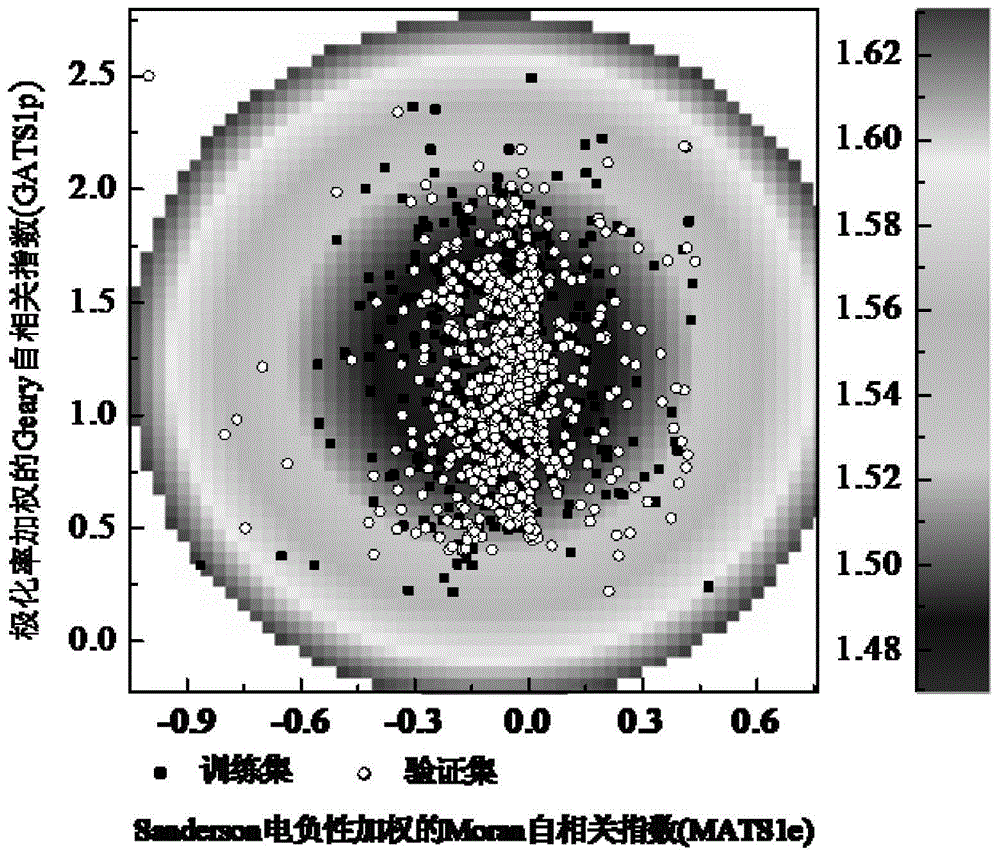
[0028] Given the compound 4-aminopyridine (SMILES:Nc1ccncc1), predict its biodegradability. First, according to the molecular structure of 4-aminopyridine, use Draogon software (Version6.0) to calculate 14 kinds of descriptors nN, nHM, O%,
[0029] The values of MATS1e, GATS1p, GATS7p, GGI1, GGI2, nCq, nCrt, C-040, H-048, H-051 and O-059 are 2, 0, 0.246, 0.914, 0, 1, 0.444, 0, respectively 0,0,0,0 and 0. According to the formula (2), the Euclidean distance of the eigenvector is 0.399 (<1.628). Within the scope of the model application domain, the model can be used to predict the biodegradability of 4-aminopyridine. Substituting the descriptor values into the built model has:
[0030] z=1.9025+1.0457×2+0.6662×0-0.1078×0+2.8362×(-0.246)-2.0019×0.914-0.7015×0+0.1131×1+0.7023×0.444+2.7793×0+1.035×0-0.777×0 -0.7091×0-0.1553×0+0.955×0=-2.961
[0031] but The biodegradability of 4-aminopyridine was predicted to be difficult to degrade, which was consistent with the experime...
Embodiment 2
[0033] Given the compound 4-methoxyphenol (SMILES: O(c(ccc(O)c1)c1)C), the values of 14 descriptors calculated by Draogon software are 0, 0, 11.8, -0.11, 1.114 , 0.528, 2, 0.889, 0, 0, 0, 0, 0, and 0. The Euclidean distance of the eigenvector calculated according to the molecular structure descriptor value is 0.219 (<1.628). Within the scope of the model application domain, the model can be used to predict the biodegradability of 4-methoxyphenol. The obtained descriptor values were substituted into the model to obtain f(z)=0.193<0.500, and the biodegradability of 4-methoxyphenol was predicted to be easy to degrade, which was consistent with the experimental results.
Embodiment 3
[0035] Given the compound bromopentane (SMILES:CCCCCBr), predict its biodegradability. The 14 descriptor values were calculated using Draogon software as 0, 1, 0, -0.015, 0.921, 0, 0.5, 0.222, 0, 0, 0, 0, 0 and 0. The Euclidean distance of the bromopentane eigenvector calculated according to the molecular structure descriptor value is 0.351 (<1.628), which is within the scope of the model application domain, so this model can be used to predict the biodegradability of bromopentane. Substituting the resulting descriptor values into the model yields
[0036] f(z)=0.710>0.500, the biodegradability of pentane bromide is predicted to be difficult to degrade, which is consistent with the experimental results.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com